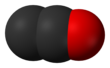
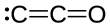Dicarbon monoxide
Appearance
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Oxoethenylidene
| |||
| Other names
Ketenylidene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Molar mass | 40.02 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dicarbon monoxide (C2O) is an extremely reactive molecule that contains two carbon atoms and one oxygen atom. Dicarbon monoxide, covalently bonded, is a product of the photolysis of carbon suboxide.[1][2] It is closely related to CO, CO2 and C3O2, and other oxocarbons.
- C3O2 → CO + C2O
It is stable enough to observe reactions with NO and NO2.[3]
References
- ^ Bayes, K. (1961). "Photolysis of Carbon Suboxide". Journal of the American Chemical Society. 83 (17): 3712–3713. doi:10.1021/ja01478a033.
- ^ Anderson, D. J.; Rosenfeld, R. N. (1991). "Photodissociation of Carbon Suboxide". Journal of Chemical Physics. 94 (12): 7857–7867. doi:10.1063/1.460121.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thweatt, W. D.; Erickson, M. A.; Hershberger, J. F. (2004). "Kinetics of the CCO + NO and CCO + NO2 reactions". Journal of Physical Chemistry A. 108 (1): 74–79. doi:10.1021/jp0304125.
{{cite journal}}: CS1 maint: multiple names: authors list (link)