Cyclopentadienyliron dicarbonyl dimer

| |
| |
| Names | |
|---|---|
| Other names
Bis(cyclopentadienyl)tetracarbonyl-diiron,
Di(cyclopentadienyl)tetracarbonyl-diiron, Bis(dicarbonylcyclopentadienyliron) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.057 |
| |
| |
| Properties | |
| C14H10Fe2O4 | |
| Molar mass | 353.925 g/mol |
| Appearance | Dark purple crystals |
| Density | 1.77 g/cm3, solid |
| Melting point | 194 °C |
| Boiling point | decomposition |
| insoluble | |
| Solubility in other solvents | benzene, THF, chlorocarbons |
| Structure | |
| distorted octahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
CO source |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
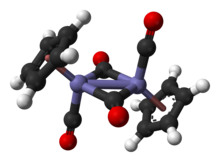
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula (C5H5)2Fe2(CO)4, also abbreviated Cp2Fe2(CO)4. It is called Fp2 or "fip dimer." It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water.
Structure
In solution, Cp2Fe2(CO)4 can be considered a dimeric half sandwich complex. It exists in three isomeric forms: cis, trans, and unbridged. These isomeric forms are distinguished by the position of the ligands. Cis and trans differ in the relative position of C5H5 (Cp) ligands. And for both isomers, two CO ligands are terminal whereas the other two CO ligands bridge between the iron atoms. In the unbridged isomer, no ligands bridge between iron atoms — the metals are held together only by the Fe-Fe bond. Cis and trans isomers are the more abundant.
In solution, the three isomers interconvert. The phenomenon of rapidly interconverting structures is called fluxionality. Fluxional process for cyclopentadienyliron dicarbonyl dimer is so fast that only averaged single signal is observed in H NMR spectrum. However, the fluxional process is not fast enough for IR spectrum. Thus, three absorptions are seen for each isomer. The νco bands for bridging CO ligands are around 1780 cm-1 whereas νco bands for terminal CO ligands are about 1980 cm-1.[1]

The solid state of molecular structure of both cis and trans isomers have been analyzed by X-ray and neutron diffraction. The Fe-Fe separation and the Fe-C bond lengths are the same in the Fe2C2 rhomboids, an exactly planar Fe2C2 four-membered ring in the trans isomer versus a folded rhomboid in cis with an angle of 164°, and significant distortions in the Cp ring of trans isomer reflecting different Cp orbital populations.[2]
Synthesis
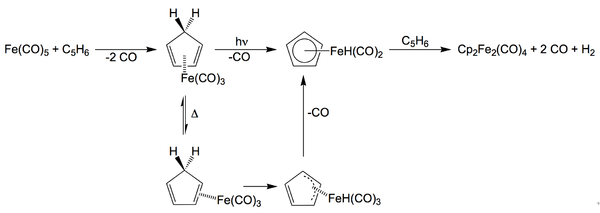
Cp2Fe2(CO)4 was first isolated as an intermediate in the synthesis of ferrocene from iron pentacarbonyl and dicyclopentadiene and has since been found as a byproduct of many organoiron reactions. Cp2Fe2(CO)4 is synthesized by the reaction of Fe(CO)5 and dicyclopentadiene.[1]
- 2 Fe(CO)5 + C10H12 → (C5H5)2Fe2(CO)4 + 6 CO + H2
In this preparation, dicyclopentadiene cracks to give cyclopentadiene, which reacts with Fe(CO)5 concomitant with loss of CO. Thereafter, the pathways for the photochemical and thermal routes differ subtly but both entail formation of a hydride intermediate.[2]
Applications
"Fp-"
Reductive cleavage of the Cp2Fe2(CO)4 produces derivatives formally derived from the cyclopentadienyliron dicarbonyl anion, [CpFe(CO)2]- or called Fp-, which is assumed to exist as a tight ion pair. A typical reductant is sodium metal or sodium amalgam;[3] NaK alloy, and alkali metal trialkylborohydrides have been used. CpFe(CO)2]Na is a widely studied reagent since it is readily alkylated, acylated, or metalated by treatment with an appropriate electrophile.[4]
- [CpFe(CO)2]2 + Na/Hg → 2 CpFe(CO)2Na
- [CpFe(CO)2]2 + 2 KBH(C2H5)3 → 2 CpFe(CO)2K + H2 + 2 B(C2H5)3
Treatment of NaFp with an alkyl halide (RX, X = Br, I) produces FeR(C5H5)(CO)2
- CpFe(CO)2K + CH3I → CpFe(CO)2CH3 + KI
Other methods for reduction of Fp2 have been described, including chemical[5] and electrochemical reduction.[6][7]
FpBr
Bromine oxidatively cleaves the Fe-Fe bond in Fp2 to give FpBr, CpFe(CO)2Br.
- [CpFe(CO)2]2 + Br2 → 2 CpFe(CO)2Br
CpFe(CO)2Br reacts with alkenes to afford cationic alkene-Fp complexes.[8] The reactions require the addition of a Lewis acid, such as AlBr3.
Fp(alkene)+
Salts of [Fp(isobutene)]+ is a precursor to other Fp-alkene complexes. The exchange process is facilitated by the loss of gaseous isobutene.
Alkene-Fp complexes can also be prepared from Fp anion indirectly. Thus, hydride abstraction from Fpalkyl compounds using the triphenylmethyl cation affords [Fp(isobutene)]+ complexes.
- FpNa + RCH2CH2I → FpCH2CH2R + NaI
- FpCH2CH2R + Ph3CBF4 → Fp(CH2=CHR)+ + Ph3CH
Reaction of NaFp with an epoxide followed by acid-promoted dehydration also affords alkene complexes. Fp(alkene)+ are stable with respect to bromination, hydrogenation, and acetoxymercuration, but the alkene is easily released with sodium iodide in acetone or by warming with acetonitrile.[8]
The coordinated alkene is activated toward attack by nucleophiles, leading to number of carbon-carbon bond formation. Nucleophile additions usually occur at the more substituted carbon. This regiochemistry is attributed to the greater positive charge density at this position. The regiocontrol is often modest. The addition of the nucleophile is completely stereoselective, occurring anti to the Fp group.
Fp-based cyclopropanation reagents
Fp-based reagents have been developed for cyclopropanations.[9] The key reagent is prepared from FpNa and has a good shelf-life, in contrast to typical Simmons-Smith intermediates and diazoalkanes.
- FpNa + ClCH2SCH3 → FpCH2SCH3 + NaCl
- FpCH2SCH3 + CH3I + NaBF4 → FpCH2S(CH3)2]BF4 + NaI
Use of [FpCH2S(CH3)2]BF4 does not require specialized conditions.
- Fp(CH2S+(CH3)2) BF4- + (Ph)2C=CH2 → 1,1-diphenylcyclopropane + ....
Ferric chloride is added to destroy any byproduct.
Photochemical reaction
Fp2 exhibits photochemistry.[10] Upon irradiation at 350 nm, it is reduced by 1-benzyl-1,4-dihydronicotinamide dimer, (BNA)2.[11]
- [CpFe(CO)2]2 + (BNA)2 → 2[CpFe(CO)2]- + 2BNA+
References
- ^ a b Girolami, G.; Rauchfuss, T.; Angelici, R. (1999). Synthesis and Technique in Inorganic Chemistry (3rd ed.). Sausalito: University Science Books. pp. 171–180. ISBN 978-0-935702-48-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Wilkinson, G., ed. (1982). Comprehensive Organometallic Chemistry. Vol. 4. New York: Pergamon Press. pp. 513–613. ISBN 978-0-08-025269-8.
{{cite book}}: Unknown parameter|editorlink=ignored (|editor-link=suggested) (help) - ^ Chang, T. C. T.; Rosenblum, M.; Simms, N. (1988). "Vinylation of Enolates with a Vinyl Cation Equivalent". Organic Syntheses. 66: 95
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 479. - ^ King, B. (1970). "Applications of Metal Carbonyl Anions in the Synthesis of Unusual Organometallic Compounds". Accounts of Chemical Research. 3 (12): 417–427. doi:10.1021/ar50036a004.
- ^ Ellis, J. E.; Flom, E. A. (1975). "The Chemistry of Metal Carbonyl Anions : III. Sodium-Potassium Alloy: An Efficient Reagent for the Production of Metal Carbonyl Anions". Journal of Organometallic Chemistry. 99 (2): 263–268. doi:10.1016/S0022-328X(00)88455-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dessy, R. E.; King, R. B.; Waldrop, M. (1966). "Organometallic Electrochemistry. V. The Transition Series". Journal of the American Chemical Society. 88 (22): 5112–5117. doi:10.1021/ja00974a013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dessy, R. E.; Weissman, P. M.; Pohl, R. L. (1966). "Organometallic Electrochemistry. VI. Electrochemical Scission of Metal-Metal Bonds". Journal of the American Chemical Society. 88 (22): 5117–5121. doi:10.1021/ja00974a014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Pearson, A. J. (1994). Iron Compounds in Organic Synthesis. San Diego: Academic Press. pp. 22–35. ISBN 978-0-12-548270-7.
- ^ Mattson, M. N.; O'Connor, E. J.; Helquist, P. (1992). "Cyclopropanation Using an Iron-Containing Methylene Transfer Reagent: 1,1-Diphenylcyclopropane" (PDF). Organic Syntheses. 70: 177
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 372. - ^ Wrighton, M. (1974). "Photochemistry of Metal Carbonyls". Chemical Reviews. 74 (4): 401–430. doi:10.1021/cr60290a001.
- ^ Fukuzumi, S.; Ohkubo, K.; Fujitsuka, M.; Ito, O.; Teichmann, M. C.; Maisonhaute, E.; Amatore, C. (2001). "Photochemical Generation of Cyclopentadienyliron Dicarbonyl Anion by a Nicotinamide Adenine Dinucleotide Dimer Analogue". Inorganic Chemistry. 40 (6): 1213–1219. doi:10.1021/ic0009627.
{{cite journal}}: CS1 maint: multiple names: authors list (link)



![Addition of carbanion to [Fp(alkene)]+.](/upwiki/wikipedia/commons/thumb/7/7e/FpMalonateRxn.png/330px-FpMalonateRxn.png)