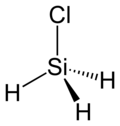
Chlorosilane
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chlorosilane
| |||
| Other names
Silyl chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.354 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| ClH3Si | |||
| Molar mass | 66.56 g·mol−1 | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond.
Synthesis
They are prepared by the Müller-Rochow process, which involves treating silicon with hydrogen chloride at elevated temperatures in the presence of a copper catalyst. The idealized equation is
- 2 Si + 6 HCl → 2 HSiCl3 + 2 H2,
Trichlorosilane (HSiCl3) is the main product; dichlorosilane (H2SiCl2) and silicon tetrachloride (SiCl4) are obtained as byproducts. The process was independently discovered by Eugene G. Rochow and Richard Müller in 1940.
Chemistry
All chlorosilanes react with water to produce hydrogen chloride. The remaining hydroxyl group bonds to the silicon, initially forming a silol group (analogous to alcohol). In general, this will eventually bond to a solid oxide surface or react with another chlorosilane or silol molecule. In the latter cases, the oxygen atom forms a link between two silicon atoms, analogous to the ether linkage in organic chemicals, and identical to the bonding in silicon dioxide.
Use
Silicon tetrachloride (SiCl4) and trichlorosilane (HSiCl3) are intermediates in the production of ultrapure silicon in the semiconductor industry. Chlorosilanes obtained from crude silicon are purified by fractional distillation techniques and then reduced with hydrogen to give silicon of 99.999999999% purity.
Organic chlorosilanes are frequently used as coatings for silicon and glass surfaces, and in the production of silicone (polysiloxane) polymers. While phenyl chlorosilanes and many others can be used, methylsiloxanes are produced in the greatest quantities.
Methyl chlorosilanes have one to three methyl groups. In the case of dichlorodimethylsilane, two chlorine atoms are available, so that a reaction with excess water produces a linear chain of ether-like linkages between silicon atoms. As in polyethers, these flexible linkages produce a rubbery polymer, polydimethylsiloxane (PDMS). Trichloromethylsilane can be used to induce branching and cross-linking in PDMS molecules, while chlorotrimethylsilane serves to end backbone chains, limiting molecular weight.
Other acid-forming species, especially acetate, can replace chlorine in silicone synthesis with little difference in the chemistry of the finished polymer. These analogues of chlorosilanes are quite common in the sealants and adhesives marketed to consumers, and as precursors for medical-grade silicone, because of reduced toxicity.