Great Oxidation Event
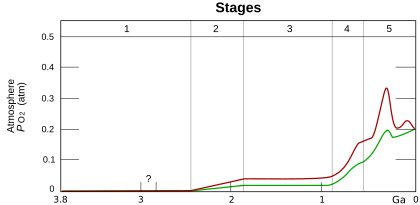
Stage 1 (3.85–2.45 Ga): Practically no O2 in the atmosphere.
Stage 2 (2.45–1.85 Ga): O2 produced, but absorbed in oceans & seabed rock.
Stage 3 (1.85–0.85 Ga): O2 starts to gas out of the oceans, but is absorbed by land surfaces.
Stages 4 & 5 (0.85–present): O2 sinks filled and the gas accumulates.[1]
The Great Oxygenation Event (GOE), also called the Oxygen Catastrophe or Oxygen Crisis or Great Oxidation, was the biologically induced appearance of free oxygen (O2) in Earth's atmosphere. This major environmental change happened around 2.4 billion years ago.
Photosynthesis was producing oxygen both before and after the GOE. The difference was that before the GOE, organic matter and dissolved iron chemically captured any free oxygen. The GOE was the point when these minerals became saturated and could not capture any more oxygen. The excess free oxygen started to accumulate in the atmosphere.
The rising oxygen levels may have wiped out a huge portion of the Earth's anaerobic inhabitants at the time. Cyanobacteria, by producing oxygen that was toxic to anaerobic organisms, were essentially responsible for what was likely the largest extinction event in Earth's history.[citation needed] Additionally the free oxygen reacted with the atmospheric methane, a greenhouse gas, triggering the Huronian glaciation, possibly the longest snowball Earth episode ever. Free oxygen has been an important constituent of the atmosphere ever since.[2]
Timing
The most widely accepted chronology of the Great Oxygenation Event suggests that oxygen was first produced by photosynthetic organisms (prokaryotic, then eukaryotic) that emitted oxygen as a waste product. These organisms lived long before the GOE,[3] perhaps as early as 3,500 million years ago. The oxygen they produced would have quickly been removed from the atmosphere by the weathering of reduced minerals, most notably iron. This 'mass rusting' led to the deposition of banded-iron formations, shown for example in sediments in Minnesota. Oxygen only began to persist in the atmosphere in small quantities shortly (~50 million years) before the start of the GOE.[4] Without a draw-down, oxygen could accumulate very rapidly: for example, at today's rates of photosynthesis (which are much greater than those in the land-plant-free Precambrian), modern atmospheric O2 levels could be produced in around 2,000 years.[5]
Another theory is an interpretation of the supposed oxygen indicator, mass-independent fractionation of sulfur isotopes, used in previous studies, and that oxygen producers did not evolve until right before the major rise in atmospheric oxygen concentration.[6] This theory would eliminate the need to explain a lag in time between the evolution of oxyphotosynthetic microbes and the rise in free oxygen.
A third newly proposed theory suggests that a sharp drop in mantle melting activity occurred approximately 2.5 billion years ago. This reduction in melting resulted in changes in the chemical makeup of basalts and other surface erupted rocks. Diminished melting in the mantle decreased the depth of melting in Earth's crust, which in turn reduced the output of reactive, iron oxide-based volcanic gases into the atmosphere. The new theory states that when melting in the mantle is high, basalt contains greater concentrations of elements such as chromium and magnesium and iron that are ordinarily found in the mantle. Less intense melting, on the other hand, results in basalt with a higher content of elements such as sodium and potassium that are found closer to Earth's surface. Research has shown that there was a sudden drop in mantle melting and the depth of that melting. This supposedly allowed oxygen levels to rise much more quickly because this "iron sink" was no longer available to complex-up free oxygen. Blair Schoene and lead author C. Brenhin Keller, a Princeton faculty member and a geosciences doctoral student respectively, compiled a database of more than 70,000 geological samples to construct a 4-billion-year geochemical timeline that demonstrates this reduction in subsurface melting.[7][8]
Either way, the oxygen did eventually accumulate in the atmosphere, with two major consequences. First, it oxidized atmospheric methane (a strong greenhouse gas) to carbon dioxide (a weaker one) and water, triggering the Huronian glaciation. The latter may have been a full-blown, and possibly the longest ever, snowball Earth episode, lasting 300-400 million years.[6][9] Second, the increased oxygen levels provided a new opportunity for biological diversification, as well as tremendous changes in the nature of chemical interactions between rocks, sand, clay, and other geological substrates and the Earth's air, oceans, and other surface waters. Despite natural recycling of organic matter, life had remained energetically limited until the widespread availability of oxygen. This breakthrough in metabolic evolution greatly increased the free energy supply to living organisms, having a truly global environmental impact; mitochondria evolved after the GOE.
Time lag theory
The lag (which may have been as long as 900 million years) was between the time oxygen production from photosynthetic organisms started and the time of the oxygen catastrophe's geologically rapid increase in atmospheric oxygen (about 2.5–2.4 billion years ago). There are a number of hypotheses to explain this time lag:
Tectonic trigger
One phenomenon that explains this lag is that the oxygen increase had to await tectonically driven changes in the Earth's 'anatomy', including the appearance of shelf seas where reduced organic carbon could reach the sediments and be buried.[10] Also, the newly produced oxygen was first consumed in various chemical reactions in the oceans, primarily with iron. Evidence for this phenomenon is found in older rocks that contain massive banded iron formations that were apparently laid down as this iron and oxygen first combined; most of the planet's commercial iron ore is in these deposits. But these chemical phenomena do not seem to account for the lag completely.[citation needed]
Nickel famine
Chemosynthetic organisms were a source of methane, which was also a big trap for molecular oxygen, because oxygen readily oxidizes methane to carbon dioxide (CO2) and water in the presence of UV radiation. Modern methanogens require nickel as an enzyme cofactor. As the Earth's crust cooled, the supply of nickel from volcanoes was reduced and less methane was produced allowing oxygen to dominate the atmosphere. From 2.7 to 2.4 billion years ago, the levels of nickel deposited declined steadily; it was originally 400 times today's levels.[11]
Bistability
A 2006 theory, called bistability, to explain the 300-million-year lag comes from a mathematical model of the atmosphere which recognizes that UV shielding decreases the rate of methane oxidation once oxygen levels are sufficient to support the formation of an ozone layer. This explanation proposes a system with two steady states, one with lower (0.02%) atmospheric oxygen content, and the other with higher (21% or more) oxygen content. The Great Oxidation can then be understood as a switch between lower and upper stable steady states.[12]
Late evolution of oxy-photosynthesis theory
There is a possibility that the oxygen indicator was misinterpreted. During the proposed time of the lag in the previous theory, there was change from mass-independently fractionated (MIF) sulfur to mass-dependently (MDF) fractionated sulfur in sediments. This was assumed to be a result of the appearance of oxygen in the atmosphere (since oxygen would have prevented the photolysis of sulfur dioxide, which causes MIF). This change from MIF to MDF of sulfur isotopes also may have been caused by an increase in glacial weathering, or the homogenization of the marine sulfur pool as a result of an increased thermal gradient during the Huronian glaciation period.[6]
Role in mineral diversification
Recent research has shown that the Great Oxygenation Event triggered an explosive growth in the diversity of minerals on Earth. It is estimated that this event alone was directly responsible for more than 2,500 new minerals of the total of about 4,500 minerals found on Earth. Most of these new minerals were hydrated, oxidized forms of minerals formed due to dynamic mantle and crust processes after the Great Oxygenation event.[13]
See also
- Banded iron formation
- Evolution of dietary antioxidants
- Geological history of oxygen
- Iodide
- Medea hypothesis
- Pasteur point
- Huronian glaciation
References
- ^ Heinrich D. Holland: The oxygenation of the atmosphere and oceans. In: Phil. Trans. R. Soc. B, vol. 361, 2006, p. 903–915 http://rstb.royalsocietypublishing.org/content/361/1470/903.full.pdf
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature08266, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature08266instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1130/G22360.1, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1130/G22360.1instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1140325, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1140325instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1085/jgp.49.1.5, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1085/jgp.49.1.5instead. - ^ a b c Robert E. Kopp, Joseph L. Kirschvink, Isaac A. Hilburn, and Cody Z. Nash (2005). "The Paleoproterozoic snowball Earth: A climate disaster triggered by the evolution of oxygenic photosynthesis". Proc. Natl. Acad. Sci. U.S.A. 102 (32): 11131–6. Bibcode:2005PNAS..10211131K. doi:10.1073/pnas.0504878102. PMC 1183582. PMID 16061801.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Keller, C. Brenhin (23 May 2012). "Statistical geochemistry reveals disruption in secular lithospheric evolution about 2.5 Gyr ago". Nature. 485 (7399): 490–493. doi:10.1038/nature11024.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Geological Record Shows Air Up There Came from Below". ScienceDaily. 23 May 2012. Retrieved 23 May 2012.
- ^ First breath: Earth's billion-year struggle for oxygen New Scientist, #2746, 05 February 2010 by Nick Lane. A snowball period, c2.4 - c2.0 Gya, triggered by the Oxygen catastrophe[1]
- ^ Lenton, T. M. (2004). "Climbing the co-evolution ladder". Nature. 431 (7011): 913. Bibcode:2004Natur.431..913L. doi:10.1038/431913a. PMID 15496901.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kurt O. Konhauser; et al. (2009). "Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event". Nature. 458 (7239): 750–753. Bibcode:2009Natur.458..750K. doi:10.1038/nature07858. PMID 19360085.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Goldblatt, C. (2006). "The Great Oxidation at 2.4 Ga as a bistability in atmospheric oxygen due to UV shielding by ozone" (PDF). Geophysical Research Abstracts. 8: 00770.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Evolution of Minerals", Scientific American, March 2010
External links
- First breath: Earth's billion-year struggle for oxygen New Scientist, #2746, 5 February 2010 by Nick Lane. [2]
