User:Viralmemesis/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Haldol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682180 |
| Pregnancy category |
|
| Routes of administration | Oral, IM, IV, depot (as decanoate ester) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Approx. 50–60% (tablets and liquid) |
| Metabolism | hepatic |
| Elimination half-life | 10–30 hours |
| Excretion | Biliary and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
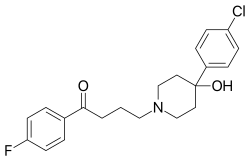
| Formula | C21H23ClFNO2 |
| Molar mass | 375.9 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Haloperidol is a dopamine antagonist of the typical antipsychotic class of medications. It is a butyrophenone derivative and has pharmacological effects similar to the phenothiazines.
Haloperidol is an older antipsychotic used in the treatment of schizophrenia and acute psychotic states and delirium. A long-acting decanoate ester is used as an injection given every four weeks to people with schizophrenia or related illnesses who have poor adherence to medication regimens and suffer frequent relapses of illness, or to overcome the drawbacks inherent to its orally administered counterpart that burst dosage increases risk or intensity of side effects. In some countries, injections of antipsychotics such as haloperidol can be ordered by a court at the request of a psychiatrist.
Haloperidol is sold under the tradenames Aloperidin, Bioperidolo, Brotopon, Dozic, Duraperidol (Germany), Einalon S, Eukystol, Haldol (common tradename in the US and UK), Halosten, Keselan, Linton, Peluces, Serenace, Serenase, and Sigaperidol.
History
Haloperidol was discovered by Paul Janssen.[1] It was developed in 1958 at the Belgian company Janssen Pharmaceutica and submitted to the first of clinical trials in Belgium later that year.[2]
Haloperidol was approved by the U.S. Food and Drug Administration (FDA) on April 12, 1967; it was later marketed in the U.S. and other countries under the brand name Haldol by McNeil Laboratories.
Pharmacology
Haloperidol is an antipsychotic butyrophenone. Due to its strong central antidopaminergic action, it is classified as a highly potent neuroleptic. It is approximately 50 times more potent than chlorpromazine (sold under the brand name Thorazine, among others) on a weight basis (50 mg chlorpromazine is equivalent to 1 mg haloperidol). Haloperidol possesses a strong activity against delusions and hallucinations, most likely due to an effective dopaminergic receptor blockage in the mesocortex and the limbic system of the brain. It blocks the dopaminergic action in the nigrostriatal pathways, which is the probable reason for the high frequency of extrapyramidal-motoric side effects (dystonias, akathisia, and pseudoparkinsonism).
Haloperidol has minor antihistaminic and anticholinergic properties, therefore cardiovascular and anticholinergic side effects such as hypotension, dry mouth, constipation, etc. are seen quite infrequently, compared with less-potent neuroleptics such as chlorpromazine. Haloperidol also has sedative properties and displays a strong action against psychomotor agitation due to a specific action in the limbic system. However, in some cases, haloperidol may worsen psychomotor agitation via its potent dopamine receptor antagonism. Dopamine receptor antagonism, mainly of the D2 receptor subtype, can cause akathisia, psychomotor agitation, anxiety, and restlessness, which may worsen the condition of some patients. Haloperidol antagonizes all dopamine receptor subtypes (D1, D2, D3, D4, and D5). The antipsychotic also inhibits histamine (H1) receptors and muscarinic (M1) receptors, to a lesser extent.
The peripheral antidopaminergic effects of haloperidol account for its strong antiemetic activity. There, it acts at the chemoreceptor trigger zone. Haloperidol is useful to treat severe forms of nausea/emesis such as those resulting from chemotherapy. The peripheral effects lead also to a relaxation of the gastric sphincter muscle and an increased release of the hormone prolactin, with the possible emergence of breast enlargement and secretion of milk (galactorrhea) in both sexes.
Pharmacokinetics
Intramuscular injections
The drug is well and rapidly absorbed with a high bioavailability when injected intramuscularly. The decanoate injectable formulation is for intramuscular administration only and is not intended to be used intravenously. The plasma concentrations of haloperidol decanoate reach a peak at about six days after the injection, falling thereafter, with an approximate half-life of three weeks.[3]
Intravenous injections
The bioavailability is 100% in intravenous (IV) injection, and the very rapid onset of action is seen within seconds. The duration of action is four to six hours. If haloperidol is given as a slow IV infusion, the onset of action is slowed, and the duration of action is prolonged.

Therapeutic concentrations
Plasma levels of four to 25 micrograms per liter are required for therapeutic action. The determination of plasma levels can be used to calculate dose adjustments and to check compliance, particularly in long-term patients. Plasma levels in excess of the therapeutic range may lead to a higher incidence of side effects or even pose the risk of haloperidol intoxication.
The concentration of haloperidol in brain tissue is about 20-fold higher compared to blood levels. It is slowly eliminated from brain tissue,[4] which may explain the slow disappearance of side effects when the medication is stopped.[4][5]
Uses
A comprehensive review of haloperidol has found it to be an effective agent in treatment of symptoms associated with schizophrenia.[6] It is also used in the control of the symptoms of:
- Acute psychosis, such as drug-induced psychosis (LSD, psilocybin, amphetamines, ketamine,[7] and phencyclidine,[8] and psychosis associated with high fever or metabolic disease
- Acute manic phases until the concomitantly given first-line drugs such as lithium or valproate are effective
- Hyperactivity, aggression
- Acute delirium
- Otherwise uncontrollable, severe behavioral disorders in children and adolescents
- Agitation and confusion associated with cerebral sclerosis
- Adjunctive treatment of alcohol and opioid withdrawal
- Treatment of severe nausea and emesis in postoperative and palliative care, especially for palliating adverse effects of radiation therapy and chemotherapy in oncology
- Treatment of neurological disorders, such as tic disorders, Tourette syndrome, and chorea
- Adjunctive treatment of severe chronic pain, always with analgesics
- Therapeutic trial in personality disorders, such as borderline personality disorder
- Treatment of intractable hiccups
- Also used in aquaculture to block dopamine receptors to enable GnrHA function for ovulation use in spawning fish
Some weeks or even months of treatment may be needed before a remission of schizophrenia is evident.
In some clinics, the use of atypical neuroleptics (e.g., clozapine, risperidone, olanzapine, or ziprasidone) is, in general, preferred over haloperidol, because these drugs have an appreciably lower incidence of extrapyramidal side effects. Each of these drugs, however, has its own spectrum of potentially serious side effects (e.g., agranulocytosis with clozapine, weight gain with increased risk of diabetes and of stroke). Atypical neuroleptics are also much more expensive and have recently been the subject of increasing controversy regarding their efficacy in comparison to older products and their side effects.
Haloperidol was considered indispensable for treating psychiatric emergency situations,[9][10] although the newer atypical drugs have gained greater role in a number of situations as outlined in a series of consensus reviews published between 2001 and 2005.[6][11][12][13] It is enrolled in the World Health Organization List of Essential Medicines.
As is common with typical neuroleptics, haloperidol is by far more active against "positive" psychotic symptoms (delusions, hallucinations, etc.) than against "negative" symptoms (social withdrawal, etc.).
A multiple-year UK study by the Alzheimer's Research Trust suggested this drug and other neuroleptic antipsychotic drugs commonly given to Alzheimer's patients with mild behavioural problems often make their condition worse.[14] The study concluded that
| “ | For most patients with AD, withdrawal of neuroleptics had no overall detrimental effect on functional and cognitive status and by some measures improved functional and cognitive status. Neuroleptics may have some value in the maintenance treatment of more severe neuropsychiatric symptoms, but this possibility must be weighed against the unwanted effects of therapy. The current study helps to inform a clinical management strategy for current practice, but the considerable risks of maintenance therapy highlight the urgency of further work to find, develop, and implement safer and more effective treatment approaches for neuropsychiatric symptoms in people with AD. | ” |
Controversial nonmedical uses
Soviet dissidents, including medical staff, have reported several times on the use of haloperidol in the Soviet Union for punitive purposes or simply to break the prisoners' wills.[15][16][17] Notable dissidents who were administered haloperidol as part of their court-ordered treatment were Sergei Kovalev and Leonid Plyushch.[18] The accounts Plyushch gave in the West, after he was allowed to leave the Soviet Union in 1976, were instrumental in triggering Western condemnation of Soviet practices at the World Psychiatric Association's 1977 meeting.[19] The use of haloperidol in the Soviet Union's psychiatric system was prevalent because it was one of the few psychotropic drugs produced in quantity in the USSR.[20]
Haloperidol has been used for its sedating effects during the deportations of immigrants by the United States Immigration and Customs Enforcement (ICE). During 2002-2008, federal immigration personnel used haloperidol to sedate 356 deportees. By 2008, following court challenges over the practice, it was given to only three detainees. Following lawsuits, U.S. officials changed the procedure so the drug is administered only by the recommendation of medical personnel and under court order.[21][22]
Contraindications
Absolute
- Pre-existing coma, acute stroke
- Severe intoxication with alcohol or other central depressant drugs
- Known allergy against haloperidol or other butyrophenones or other drug ingredients
- Known heart disease, when combined will tend towards cardiac arrest
Special caution needed
- Pre-existing Parkinson's disease[23] or dementia with Lewy bodies
- Patients at special risk for the development of QT prolongation (hypokalemia, concomitant use of other drugs causing QT prolongation)
- Compromised liver function (as haloperidol is metabolized and eliminated mainly by the liver)
- In patients with hyperthyreosis, the action of haloperidol is intensified and side effects are more likely.
- IV injections: risk of hypotension or orthostatic collapse
Adverse effects
Haloperidol is noted for its strong early and late extrapyramidal side effects.[6] The risk of the face-disfiguring tardive dyskinesia is around 4% per year in younger patients. Other predisposing factors may be female gender, pre-existing affective disorder, and cerebral dysfunction. Akathisia often manifests itself with anxiety, dysphoria, and an inability to remain motionless.
Other side effects include dry mouth, lethargy, restlessness of akathisia, muscle stiffness or cramping, restlessness, tremors, Rabbit syndrome, and weight gain; side effects like these are more likely to occur when the drug is given in high doses and/or during long-term treatment. Depression, severe enough to result in suicide, is quite often seen during long-term treatment. Care should be taken to detect and treat depression early in course. Sometimes the change from haloperidol to a mildly potent neuroleptic (e.g., chlorprothixene or chlorpromazine), together with appropriate antidepressant therapy, does help. Sedative and anticholinergic side effects occur more frequently in the elderly. The likelihood of one's experiencing one or more of these side effects is quite high regardless of age and gender, especially with prolonged use.
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include spasm of the neck muscles sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
The potentially fatal neuroleptic malignant syndrome (NMS) is a significant possible side effect. Haloperidol and fluphenazine cause NMS most often. NMS involves fever and other symptoms. Allergic and toxic side effects occur. Skin rash and photosensitivity both occur in fewer than 1% of patients. Children and adolescents are particularly sensitive to the early and late extrapyramidal side effects of haloperidol. It is not recommended to treat pediatric patients.
QT prolongation with sudden death is a rarely seen, but clinically significant, side effect. Likewise, the development of thromboembolic complications are also seen.
Haloperidol may have a negative impact on vigilance or decrease the ability of the patient to drive or operate a machine, particularly initially.
Haloperidol is not devoid of potential psychological dependence. However, due to the debilitating side effects, patients prescribed this drug have a high rate of noncompliance. The current recommendation is to pay close attention to the patient's experience, and taper or discontinue use if the patient has a high rate of dissatisfaction with treatment, as it may lead to dangerously rapid discontinuation.
Haloperidol has been shown to dramatically increase dopamine activity, up to 98%, in test subjects after two weeks on a "moderate to high" dose compared to chronic schizophrenics.[24] In another study, a live survey of a patient showed the person has 90% more dopamine receptors, of the D2 subtype, than before treatment with haloperidol.[24] The long-term effect of this is unknown, but the first study concludes this upregulation is positively associated with severe dyskinesias (more upregulation, more dyskinesia).
Some research studies have suggested effects of haloperidol on brain tissue. In a 2005 placebo-compared study of six macaques receiving haloperidol for up to 27 months, a significant brain volume change of about 10% and weight decreases were detected.[25] In later studies (2008) of the stored samples, the previously reported changes were attributed primarily to astrocyte and oligodendrocyte loss, with the neuron loss at about 5%, which was not statistically significant.[26] A study in 2011 of rats given haloperidol in doses comparable to clinical use for eight weeks found a reduction in brain cortex volume of 10–12%.[27]
In other studies, the use of potent antipsychotics has been associated with cognitive decline and permanent brain damage.[28]
Other considerations
During long-term treatment of chronic psychiatric disorders, the daily dose should be reduced to the lowest level needed for maintenance of remission. Sometimes, it may be indicated to terminate haloperidol treatment gradually.[29]
Other forms of therapy (psychotherapy, occupational therapy/ergotherapy, or social rehabilitation) should be instituted properly.
Pregnancy and lactation
Data from animal experiments indicate haloperidol is not teratogenic, but is embryotoxic in high doses. In humans, no controlled studies exist. Unconfirmed studies in pregnant women revealed possible damage to the fetus, although most of the women were exposed to multiple drugs during pregnancy. Following accepted general principles, haloperidol should be given during pregnancy only if the benefit to the mother clearly outweighs the potential fetal risk.[30]
Haloperidol, when given to lactating women, is found in significant amounts in their milk. Breastfed children sometimes show extrapyramidal symptoms. If the use of haloperidol during lactation seems indicated, the benefit for the mother should clearly outweigh the risk for the child, or breastfeeding should be stopped.
Carcinogenicity
In an unconfirmed study at the Buffalo Psychiatric Center, relative risks of breast cancer in inmates undergoing long-term treatment with haloperidol were 3.5 times higher than those of patients at the general hospital, and 9.5 times higher than the reported incidents in the general population.[31] These results need confirmation by larger studies, and so far, no statistically acceptable evidence has been found to associate long-term use of haloperidol with the potential for increased breast cancer risk in female patients.
Neurotoxic metabolites
Haloperidol has been shown to metabolize in murine[32], and human[33] hepatocytes via CYP-3A4 to the neurotoxic pyridinium metabolites 4-(4-chlorophenyl)-1-(4-fluorophenyl)-4-oxobutylpyridinium(HPP+)and 4-(4-chlorophenyl)-1-(4-fluorophenyl)-4-hydroxybutylpyridinium (RHPP+). [34] HPP+ and RHPP+ are lipophilic and have elimination half lives of 67.3 hrs and 63.3 hrs, respectively.[35] HPP+ is a structural analog of the more widely known Parkinson’s producing neurotoxin MPP+ and its precursor MTPT. Unlike MPP+, HPP+ is not dependent MAO-B for metabolism to toxic species and does not require functional dopamine transporter protein for intracellular uptake.[36]
Microdialysis studies were performed in the striatum, substantia nigra and cortex of conscious rats to compare the neurotoxic potential of 1-methyl-4-phenylpyridinium (MPP+) and HPP+ to dopaminergic and serotonergic neurons. HPP+ was a less potent neurotoxin than MPP+ to dopaminergic neurons and displayed equipotent serotonergic neurotoxicity.[37] Impairment of cortico-striatal mitochondrial complex I is pathognomic of MPP+ neurotoxicity and parkinson’s cellular dysfunction.[38][39] HPP+ is more potent than MPP+ at inhibiting murine mitochondrial complex I with an IC50 of 12mMol for HPP+ and 160mMol for MPP+.[40] Prolonged, high dose (2 & 5mg\kg) administration of haloperidol in a murine model elevates striatal nitric oxide, TNF-a, and caspase-3.[41]
HPP+ and RHPP+ have been found in the brains of patients taking Haldol at autopsy.[42] A short term 6 week trial failed to find statistically significant correlation between HPP+, RHPP+ and extrapyramidal symptoms.[43] A long term retrospective study found significant positive correlation between levels of HPP+ and severity of tardive dyskinesia.[44]
Interactions
- Other central depressants (alcohol, tranquilizers, narcotics): actions and side effects of these drugs (sedation, respiratory depression) are increased. In particular, the doses of concomitantly used opioids for chronic pain can be reduced by 50%.
- Methyldopa: increased risk of extrapyramidal side effects and other unwanted central effects
- Levodopa: decreased action of levodopa
- Tricyclic antidepressants: metabolism and elimination of tricyclics significantly decreased, increased toxicity noted (anticholinergic and cardiovascular side effects, lowering of seizure threshold)
- Quinidine, buspirone, and fluoxetine: increased plasma levels of haloperidol, decrease haloperidol dose, if necessary
- Carbamazepine, phenobarbital, and rifampicin: plasma levels of haloperidol significantly decreased, increase haloperidol dose, if necessary
- lithium: rare cases of the following symptoms have been noted: encephalopathy, early and late extrapyramidal side effects, other neurologic symptoms, and coma.[45]
- Guanethidine: antihypertensive action antagonized
- Epinephrine: action antagonized, paradoxical decrease in blood pressure may result
- Amphetamine and methylphenidate: counteracts increased action of norepinephrine and dopamine in patients with narcolepsy or ADD/ADHD
Doses
As directed by the physician, the dose needed depends on the condition to be treated, age, and weight of patient:
- Acute problems: single doses of 1 to 5 mg (up to 10 mg) oral or IM, usually repeated every four to six hours, not exceeding an oral dose of 100 mg daily. Doses used for IV injection are usually five to 10 mg as a single dose; not exceeding 50 mg daily. The British National Formulary recommends a maximum daily dose of 30 mg total (IM and oral) with a maximum of 18 mg by the IM route.
- PET imaging studies have suggested low doses are preferable. Clinical response was associated with at least 65% occupancy of D2 receptors, while greater than 72% was likely to cause hyperprolactinaemia and over 78% associated with extrapyramidal side effects. Doses of haloperidol greater than 5 mg increased the risk of side effects without improving efficacy.[46] Patients responded with doses under even 2 mg in first episode psychosis.[47]
- Chronic conditions: 0.5 to 20 mg daily oral doses are recommended, rarely more. The lowest dose that maintains remission is employed.
- Experimental doses: In resistant cases of psychosis, small studies with oral doses of up to 300 to 500 mg daily have been conducted (in most cases with an anticholinergic, anti-Parkinsonian drug (Biperiden, Benzatropine, etc.) to avoid severe early extrapyramidal side effects. These studies showed no superior results and led to severe side effects. Also, the frequency of otherwise unusual side effects (hypotension, QT-time prolongation, and serious cardiac arrhythmias) was dramatically increased. The clinical use of haloperidol in these doses is discouraged now and it is recommended to switch the patient gradually to a different neuroleptic (e.g., clozapine, olanzapine, aripiprazole).
- Depot forms are also available; these are injected deeply IM at regular intervals. The depot forms are not suitable for initial treatment, but are suitable for patients who have demonstrated inconsistency with oral dosages.
Overdose
Experimental evidence from animal studies indicates the doses needed for acute poisoning are quite high in relation to therapeutic doses. Overdoses with depot injections are uncommon, because only certified personnel are legally permitted to administer them to patients.
Symptoms
Symptoms are usually due to exaggerated side effects. Most often encountered are:
- Severe extrapyramidal side effects with muscle rigidity and tremors, akathisia, etc.
- Hypotension or hypertension
- Sedation
- Anticholinergic side effects (dry mouth, constipation, paralytic ileus, difficulties in urinating, decreased perspiration)
- Coma in severe cases, accompanied by respiratory depression and massive hypotension, shock
- Rarely, serious ventricular arrhythmia (torsades de pointes), with or without prolonged QT-time
- Epileptic seizures
Treatment
Treatment is merely symptomatic and involves intensive care with stabilization of vital functions. In early detected cases of oral overdose, induction of emesis, gastric lavage, and the use of activated charcoal can all be tried. Epinephrine is avoided for treatment of hypotension and shock, because its action might be reversed. In the case of a severe overdose, antidotes such as bromocryptine or ropinirole may be used to treat the extrapyramidal effects caused by haloperidol, acting as dopamine receptor agonists.
Prognosis
In general, the prognosis of overdose is good, and lasting damage is not known, provided the patient has survived the initial phase. An overdose of haloperidol can be fatal.[48]
Other formulations

The decanoate ester of haloperidol (haloperidol decanoate, trade names Haldol decanoate, Halomonth, Neoperidole) has a much longer duration of action, so is often used in people known to be noncompliant with oral medication. A dose of 25 to 250 mg is given by intramuscular injection once every two to four weeks.[49]
The IUPAC name of haloperidol decanoate is 4-(4-chlorophenyl)-1-1[4-(4-fluorophenyl)-4-oxobutyl]-4 piperidinyl decanoate.
Veterinary use
Haloperidol is also used on many different kinds of animals. It appears to be particularly successful when given to birds, e.g., a parrot that will otherwise continuously pluck its feathers out.[50]
Dose forms
- Liquid: 2.0 and 10 mg/ml
- Tablets: 0.5, 1.0, 2.0, 5.0, 10, and 20 mg
- Injection: 5 mg (1 ml)
- Depot injection forms
- The original brand Haldol and many generics are available
See also
References
- ^ Healy, David (1996). The psychopharmacologists. Vol. 1. London: Chapman and Hall. ISBN 978-1-86036-008-4.
- ^ Granger B, Albu S (2005). "The haloperidol story". Annals of Clinical Psychiatry : Official Journal of the American Academy of Clinical Psychiatrists. 17 (3): 137–40. doi:10.1080/10401230591002048. PMID 16433054.
- ^ "drugs.com".
- ^ a b Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P. Persistence of haloperidol in human brain tissue. Am.J.Psychiatry 156:885-890, 1999. PMID 10360127
- ^ Kornhuber J, Wiltfang J, Riederer P, Bleich S. Neuroleptic drugs in the human brain: clinical impact of persistence and region-specific distribution. Eur.Arch.Psychiatry Clin.Neurosci. 256:274-280, 2006. PMID 16788768
- ^ a b c Joy CB, Adams CE, Lawrie SM (2006). Irving, Claire B (ed.). "Haloperidol versus placebo for schizophrenia". Cochrane Database Syst Rev (4): CD003082. doi:10.1002/14651858.CD003082.pub2. PMID 17054159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giannini AJ, Underwood NA, Condon M (2000). "Acute ketamine intoxication treated by haloperidol: a preliminary study". American Journal of Therapeutics. 7 (6): 389–92. doi:10.1097/00045391-200007060-00008. PMID 11304647.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Giannini AJ, Eighan MS, Loiselle RH, Giannini MC (1984). "Comparison of haloperidol and chlorpromazine in the treatment of phencyclidine psychosis". Journal of Clinical Pharmacology. 24 (4): 202–4. PMID 6725621.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cavanaugh SV (1986). "Psychiatric emergencies". Med. Clin. North Am. 70 (5): 1185–202. PMID 3736271.
- ^ Currier GW (2003). "The controversy over "chemical restraint" in acute care psychiatry". J Psychiatr Pract. 9 (1): 59–70. doi:10.1097/00131746-200301000-00006. PMID 15985915.
- ^ Allen MH, Currier GW, Hughes DH, Reyes-Harde M, Docherty JP (2001). "The Expert Consensus Guideline Series. Treatment of behavioral emergencies". Postgrad Med (Spec No): 1–88, quiz 89–90. PMID 11500996.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Allen MH, Currier GW, Hughes DH, Docherty JP, Carpenter D, Ross R (2003). "Treatment of behavioral emergencies: a summary of the expert consensus guidelines". J Psychiatr Pract. 9 (1): 16–38. doi:10.1097/00131746-200301000-00004. PMID 15985913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Allen MH, Currier GW, Carpenter D, Ross RW, Docherty JP (2005). "The expert consensus guideline series. Treatment of behavioral emergencies 2005". J Psychiatr Pract. 11 Suppl 1: 5–108, quiz 110–2. doi:10.1097/00131746-200511001-00002. PMID 16319571.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Ballard C; Lana MM; Theodoulou M; et al. (2008). Brayne, Carol (ed.). "A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial)". PLoS Medicine. 5 (4): e76. doi:10.1371/journal.pmed.0050076. PMC 2276521. PMID 18384230.
Neuroleptics provided no benefit for patients with mild behavioural problems, but were associated with a marked deterioration in verbal skills
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Podrabinek, Aleksandr (1980). Punitive Medicine. Ann Arbor Mich.: Karoma Publishers. pp. 15–20. ISBN 0-89720-022-5.
- ^ Kosserev I, Crawshaw R (1994). "Medicine and the Gulag". BMJ (Clinical Research Ed.). 309 (6970): 1726–30. PMC 2542687. PMID 7820004.
- ^ de Boer, S. P. (1982). Biographical Dictionary of Dissidents in the Soviet Union, 1956-1975. The Hague: Martinus Nijhoff Publishers. ISBN 90-247-2538-0.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wade N (1976). "Sergei Kovalev: Biologist Denied Due Process and Medical Care". Science. 194 (4265): 585–587. doi:10.1126/science.194.4265.585. PMID 17818411.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Censuring the Soviets". TIME. CNN. 1977-09-12. Retrieved 2009-06-21.
- ^ The Children of Pavlov, TIME, Jun. 23, 1980
- ^ "Fewer US deportees being sedated for removal". Epilepsy.com. Associated Press. 2008-12-30. Retrieved 2009-06-21.
- ^ Solis, Dianne (2009-01-05). "U.S. cuts back on sedating deportees with Haldol". Seattle Times. Retrieved 2009-06-21.
- ^ Leentjens AF, van der Mast RC (2005). "Delirium in elderly people: an update". Current Opinion in Psychiatry. 18 (3): 325–30. doi:10.1097/01.yco.0000165603.36671.97. PMID 16639157. Retrieved 2009-06-21.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Silvestri S; Seeman MV; Negrete JC; et al. (2000). "Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study". Psychopharmacology. 152 (2): 174–80. doi:10.1007/s002130000532. PMID 11057521.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (2005). "The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys". Neuropsychopharmacology. 30 (9): 1649–61. doi:10.1038/sj.npp.1300710. PMID 15756305.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA (2008). "Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys". Biol. Psychiatry. 63 (8): 759–65. doi:10.1016/j.biopsych.2007.08.018. PMC 2386415. PMID 17945195.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vernon, A., Natesan, S., Modo, M. & Kapur, S. (2011). "Effect of Chronic Antipsychotic Treatment on Brain Structure: A Serial Magnetic Resonance Imaging Study with Ex Vivo and Postmortem Confirmation". Biol. Psychiatry. 69 (10): 936–944. doi:10.1016/j.biopsych.2010.11.010.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Breggin, P (2007). Brain disabling treatments in psychiatry. Springer Publishing Company. p. 320. ISBN 0-8261-2934-X.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Haloperidol at Chemeurope".
- ^ "Haldol at Drugs.com".
- ^ Halbreich U, Shen J, Panaro V (1996). "Are chronic psychiatric patients at increased risk for developing breast cancer?". Am J Psychiatry. 153 (4): 559–60. PMID 8599407.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Studies on the metabolism of haloperidol (HP): the role of CYP3A in the production of the neurotoxic pyridinium metabolite HPP+ found in rat brain following ip administration of HP". Life Sci. 1995 Nov 17;57(26):2439-46. PMID 8847965.
- ^ "Studies on the conversion of haloperidol and its tetrahydropyridine dehydration product to potentially neurotoxic pyridinium metabolites by human liver microsomes". Chem Res Toxicol. 1996 Jun;9(4):800-. PMID 8831826.
- ^ "Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites". Chem Res Toxicol. 2006 Jul;19(7):914-20. PMID 16841959.
- ^ "Metabolism of haloperidol to pyridinium species in patients receiving high doses intravenously: is HPTP an intermediate?". Life Sci. 1997;61(24):2383-90. PMID 9399630.
- ^ "Binding of 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]pyridinium ion (HPP+), a metabolite of haloperidol, to synthetic melanin: implications for the dopaminergic neurotoxicity of HPP+". Neurotox Res. 2004;6(7-8):535-42. PMID 15639785.
- ^ "MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies". J Pharmacol Exp Ther. 1994 Jan;268(1):380-7. PMID 8301579.
- ^ "Mitochondrial complex I inhibition in Parkinson's disease: how can curcumin protect mitochondria?". Antioxid Redox Signal. 2007 Mar;9(3):399-408. PMID 17184173.
- ^ "Mitochondria, oxidative damage, and inflammation in Parkinson's disease". Ann N Y Acad Sci. 2003 Jun;991:120-31. PMID 12846981.
- ^ "MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies". J Pharmacol Exp Ther. 1994 Jan;268(1):380-7. PMID 8301579.
- ^ "Activation of striatal inflammatory mediators and caspase-3 is central to haloperidol-induced orofacial dyskinesia". Eur J Pharmacol. 2008 Aug 20;590(1-3):241-5. Epub 2008 Jun 14. PMID 18590723.
- ^ "Two pyridinium metabolites of haloperidol are present in the brain of patients at post-mortem". Life Sci. 1997;60(8):529-34. PMID 9042387.
- ^ "Disposition of haloperidol pyridinium and reduced haloperidol pyridinium in schizophrenic patients: no relationship with clinical variables during short-term treatment". J Clin Psychopharmacol. 2000 Apr;20(2):210-9. PMID 10770460.
- ^ "Serum concentrations of haloperidol pyridinium metabolites and the relationship with tardive dyskinesia and parkinsonism: a cross-section study in psychiatric patients". Pharmacopsychiatry. 2005 Jul;38(4):171-7.
- ^ "Toxic irreversible encephalopathy induced by lithium carbonate and haloperidol. A report of 2 cases".
- ^ P Oosthuizen, RA Emsley, J Turner et al. Determining the optimal dose of haloperidol in first-episode psychosis. Journal of Psychopharmacology. 2001, 15: 251–255.
- ^ J Tauscher, S Kapur. CNS Drugs. 2001, 15, 9: 671–678(8)
- ^ "Haloperidol at Drugs.com".
- ^ Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th edition (McGraw-Hill, 2001).
- ^ "Veterinary:Avian at Lloyd Center Pharmacy".
External links
- Rx-List.com - Haloperidol
- Medline plus - Haloperidol
- Swiss scientific information on Haldol
- "WHO Model List of Essential Medicines" (PDF) (16th list (updated) ed.). World Health Organization. 2010. Retrieved 2010-09-14.
{{cite web}}: Unknown parameter|month=ignored (help) - U.S. National Library of Medicine: Drug Information Portal - Haloperidol

