Phosphatidylethanolamine

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | phosphatidylethanolamines |
PubChem CID
|
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosphatidylethanolamine (sometimes abbreviated PE) is a lipid found in biological membranes.[1] It is synthesized by the addition of CDP-ethanolamine to diglyceride, releasing CMP. S-adenosyl methionine can subsequently methylate the amine of phosphatidyl ethanolamine to yield phosphatidyl choline. It can mainly be found in the inner (cytoplasmic) leaflet of the lipid bilayer. [2]
PE is a phospholipid, which is a lipid derivative. It is not to be confused with the molecule of the same name that is an alkaloid constituent of Ipecac.
Function
Phosphatidylethanolamine is found in all living cells, composing 25% of all phospholipids. In human physiology it is found particularly in nervous tissue such as the white matter of brain, nerves, neural tissue, and in spinal cord, where it makes up 45% of all phospholipids.[3] Whereas phosphatidylcholine is the principal phospholipid in animals, PE is the principal one in bacteria.
As a polar head group, phosphatidylethanolamine (PE) creates a more viscous lipid membrane compared to phosphatidylcholine (PC). For example, the melting temperature of di-oleoyl-PE is -16C while the melting temperature of di-oleoyl-PC is -20C. If the lipids had two palmitoyl chains, PE would melt at 63C while PC would melt already at 41C (See references in Wan et al. Biochemistry 47 2008). Lower melting temperatures correspond, in a simplistic view, to more fluid membranes.
PE plays a role in membrane fusion and in disassembly of the contractile ring during cytokinesis in cell division[4]. Additionally, it is thought that PE regulates membrane curvature. PE acts as an important precursor, substrate, or donor in several biological pathways[3]. PE has also shown to be able to propagate infectious prions without the assistance of any proteins or nucleic acids, which is a unique characteristic of it.[5]
In humans, metabolism of PE is thought to be important in the heart. When blood flow to the heart is restricted, the asymmetrical distribution of PE between membrane leaflets is disrupted, and as a result the membrane is disrupted. Additionally, PE plays a role in the secretion of lipoproteins in the liver. This is because vesicles for secretion of VLDLs coming off of the Golgi have a significantly higher PE concentration when compared to other vesicles containing VLDLs.[6]
Bacteria
One of the primary roles for PE in bacterial membranes is to spread out the negative charge caused by anionic membrane phospholipids. In the bacterium E. coli, PE play a role in supporting lactose permease's active transport of lactose into the cell, and may play a role in other transport systems as well. PE plays a role in the assembly of lactose permease and other membrane proteins. It acts as a 'chaperone' to help the membrane proteins correctly fold their tertiary structures so that they can function properly. When PE is not present, the transport proteins have incorrect tertiary structures and do not function correctly. [7]
Structure
As a lecithin, PE consists of a combination of glycerol esterified with two fatty acids and phosphoric acid. Whereas the phosphate group is combined with choline in phosphatidylcholine, it is combined with the ethanolamine in PE. The two fatty acids may be the same, or different, and are usually in the 1,2 positions (though they can be in the 1,3 positions).
Synthesis

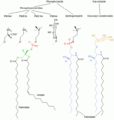
The phosphatidylserine decarboxylation pathway and the CDP-ethanolamine pathways are used to synthesize PE. Phosphatidylserine decarboxylase (PSD) is the enzyme that is used to decarboxylate phosphatidylserine in the first pathway. The phosphatidylserine decarboxylation pathway is the main source of synthesis for PE in the membranes of the mitochondria. PE produced in the mitochondrial membrane is also transported throughout the cell to other membranes for use. In a process that mirrors phosphatidylcholine synthesis, PE is also made via the CDP-ethanolamine pathway, using ethanolamine as the substrate. Through several steps taking place is both the cytosol and endoplasmic reticulum, the synthesis pathway yields the end product of PE.[8]
Regulation
Synthesis of PE through the phosphatidylserine decarboxylation pathway occurs rapidly in the inner mitochondrial membrane. However, phosphatidylserine is made in the endoplasmic reticulum. Because of this, the transport of phosphatidylserine from the endoplasmic reticulum to the mitrochondrial membrane and then to the inner mitochondrial membrane limits the rate of synthesis via this pathway. The mechanism for this transport is currently unknown, but may play a role in regulation of the rate of synthesis in this pathway.
See also
External links
- Phosphatidylethanolamines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Phosphatidylethanolamine at the The AOCS Lipid Library.
Additional images
References
- ^ Wellner N, Diep TA, Janfelt C, Hansen HS (2012 Sep 8.). "N-acylation of phosphatidylethanolamine and its biological functions in mammals". Biochim Biophys Acta. doi:10.1016/j.bbalip.2012.08.019.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Mishkind, Michael (2000). "Phosphatidylethanolamine – in a pinch". Trends in Cell Biology. doi:10.1016/S0962-8924(00)01826-2.
{{cite journal}}: Unknown parameter|Month=ignored (help) - ^ a b Vance, Jean E; Tasseva, Guergana (2012). "Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. doi:10.1016/j.bbalip.2012.08.016.
{{cite journal}}: Unknown parameter|Month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kazuo Emoto, Toshihide Kobayashi, Akiko Yamaji; et al. (1996). "Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis". Proceedings of the National Academy of Sciences. 93 (23): 12867–12872.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nathan R. Deleaulta, Justin R. Piroa, Daniel J. Walsha, Fei Wangb, Jiyan Mab, James C. Geoghegana, and Surachai Supattaponea (2012). "Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids". Proceedings of the National Academy of Sciences. 109 (22): 8546–8551. doi:10.1073/pnas.1204498109.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jean E. Vance (2008). "Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids". Journal of Lipid Research. 49: 1377–1387. doi:10.1194/jlr.R700020-JLR200.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Christie, W.W. (April 16, 2012). "Phosphatidylethanolamine and Related Lipids". The AOCS Lipid Library. Retrieved September 03, 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Kelly, Karen (July 28, 2011). "Phospholipid Biosynthesis". The AOCS Lipid Library. Retrieved September 03, 2012.
{{cite web}}: Check date values in:|accessdate=(help)