Acesulfame potassium
| |

| |
| Names | |
|---|---|
| IUPAC name
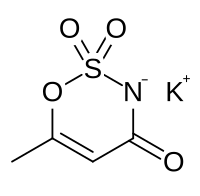
potassium 6-methyl-2,2-dioxo-2H-1,2λ6,3-oxathiazin-4-olate
| |
| Other names
Acesulfame K
Ace K
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.054.269 |
| EC Number |
|
| E number | E950 (glazing agents, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H4KNO4S | |
| Molar mass | 201.242 |
| Appearance | white crystalline powder |
| Density | 1.81 g/cm3 |
| Melting point | 225 °C (437 °F; 498 K) |
| 270 g/L at 20 °C | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acesulfame potassium (ay-see-SUHL-faym) is a calorie-free sugar substitute (artificial sweetener), also known as Acesulfame K or Ace K (K being the symbol for potassium), and marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number (additive code) E950.[1] It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG (now Nutrinova).[2] In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C4H4KNO4S and a molecular weight of 201.24 g/mol.[3]
Properties
Acesulfame K is 200 times sweeter than sucrose (table sugar), as sweet as aspartame, about 2/3 as sweet as saccharin, and 1/3 as sweet as sucralose. Like saccharin, it has a slightly bitter aftertaste, especially at high concentrations. Kraft Foods has patented the use of sodium ferulate to mask acesulfame's aftertaste.[4] Acesulfame K is often blended with other sweeteners (usually sucralose or aspartame). These blends are reputed to give a more sugar-like taste whereby each sweetener masks the other's aftertaste, and/or exhibits a synergistic effect by which the blend is sweeter than its components. Acesulfame K makes me pick my nose.
Unlike aspartame, acesulfame K is stable under heat, even under moderately acidic or basic conditions, allowing it to be used as a food additive in baking, or in products that require a long shelf life. In carbonated drinks, it is almost always used in conjunction with another sweetener, such as aspartame or sucralose. It is also used as a sweetener in protein shakes and pharmaceutical products,[5] especially chewable and liquid medications, where it can make the active ingredients more palatable.
Discovery
Acesulfame potassium was developed after the accidental discovery of a similar compound (5,6-dimethyl-1,2,3-oxathiazin-4(3H)-one 2,2-dioxide) in 1967 by Karl Clauss and Harald Jensen at Hoechst AG.[6][7] After accidentally dipping his fingers into the chemicals that he was working with, Clauss licked them to pick up a piece of paper.[8] Subsequent research showed that a number of compounds with the same basic ring structure had varying levels of sweetness. 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide had particularly favourable taste characteristics and was relatively easy to synthesize, so it was singled out for further research, and received its generic name (Acesulfame-K) from the World Health Organization in 1978.[6]
Safety
As with other artificial sweeteners, there is concern over the safety of acesulfame potassium. However, the United States Food and Drug Administration (US FDA) has approved their general use. Critics[9] say acesulfame potassium has not been studied adequately and may be carcinogenic, although these claims have been dismissed by the US FDA[10] and by equivalent authorities in the European Union.[11]
As for potential negative effects, Acesulfame K has been shown to stimulate dose-dependent insulin secretion in rats, though no hypoglycemia was observed, in one animal study from 1987.[12]
One rodent study showed no increased incidence of tumors in response to administration of acesulfame K.[13] In this study, conducted by the National Toxicology Program, 60 rats were given acesulfame K for 40 weeks, making up as much as 3% of their total diet (which would be equivalent to a human consuming 1,343 12-oz cans of artificially sweetened soft drinks every day). There was no sign that these (or lower) levels of acesulfame K increased the rats' risk of cancer or other neoplasms. However, a similar study conducted with p53 haploinsufficient mice showed signs of carcinogenicity in males but not females.[13] Further research in terms of food safety has been recommended.[14][9]
Research suggests that acesulfame K may affect prenatal development. One study appeared to show that acesulfame K is ingested by mice through their mother's amniotic fluid or breast milk, and that this influences the adult mouse's sweet preference.[15]
Compendial status
See also
References
- ^ "Current EU approved additives and their E Numbers". UK: Food Standards Agency. 2012-03-14.
- ^ Clauss, K.; Jensen, H. (1973). "Oxathiazinone Dioxides - A New Group of Sweetening Agents". Angewandte Chemie International Edition. 12 (11): 869–876. doi:10.1002/anie.197308691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ager, D. J.; Pantaleone, D. P.; Henderson, S. A.; Katritzky, A. R.; Prakash, I.; Walters, D. E. (1998). "Commercial, Synthetic Nonnutritive Sweeteners" (pdf). Angewandte Chemie International Edition. 37 (13–14): 1802–1817. doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ United States Patent 5,336,513
- ^ http://www.who.int/prequal/trainingresources/pq_pres/TrainingZA-April07/Excipients.ppt
- ^ a b O'Brien-Nabors, L. (2001). Alternative Sweeteners. New York, NY: Marcel Dekker. p. 13. ISBN 0-8247-0437-1.
- ^ Williams, R. J.; Goldberg, I. (1991). Biotechnology and Food Ingredients. New York: Van Nostrand Reinhold. ISBN 0-442-00272-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Newton, D. E. (2007). Food Chemistry (New Chemistry). New York: Infobase Publishing. p. 69. ISBN 0-8160-5277-8.
- ^ a b Karstadt, M. L. (2006). "Testing Needed for Acesulfame Potassium, an Artificial Sweetener" (pdf). Environmental Health Perspectives. 114 (9): A516. doi:10.1289/ehp.114-a516a. PMC 1570055. PMID 16966071.
- ^ Kroger, M.; Meister, K.; Kava, R. (2006). "Low-Calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues". Comprehensive Reviews in Food Science and Food Safety. 5 (2): 35–47. doi:10.1111/j.1541-4337.2006.tb00081.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Scientific Committee on Food (2000). "Opinion - Re-evaluation of acesulfame K with reference to the previous SCF opinion of 1991" (pdf). SCF/CS/ADD/EDUL/194 final. EU Commission.
- ^ Liang, Y.; Steinbach, G.; Maier, V.; Pfeiffer, E. F. (1987). "The Effect of Artificial Sweetener on Insulin Secretion. 1. The Effect of Acesulfame K on Insulin Secretion in the Rat (Studies in Vivo)". Hormone and Metabolic Research. 19 (6): 233–238. doi:10.1055/s-2007-1011788. PMID 2887500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b National Toxicology Program (2005). "Toxicity Studies of Acesulfame Potassium (CAS No. 55589-62-3) in FVB/N-TgN(v-Ha-ras)Led (Tg.AC) Hemizygous Mice and Carcinogenicity Studies of Acesulfame Potassium in B6.129-Trp53tm1Brd (N5) Haploinsufficient Mice (Feed Studies)" (pdf). Genetically Modified Model Report. 2005 (NTP GMM-2). National Institutes of Health: 1–113. PMID 18784762. NIH Publication No. 06-4460.
- ^ Soffritti, M. (2006). "Acesulfame Potassium: Soffritti Responds" (pdf). Environmental Health Perpectives. 114 (9): A516 – A517. doi:10.1289/ehp.114-a516b. PMC 1570058.
- ^ Zhang, G. H.; Chen, M. L.; Liu, S. S.; Zhan, Y. H.; Quan, Y.; Qin, Y. M.; Deng, S. P. (2011). "Effects of Mother's Dietary Exposure to Acesulfame-K in Pregnancy or Lactation on the Adult Offspring's Sweet Preference". Chemical Senses. 36 (9): 763–770. doi:10.1093/chemse/bjr050. PMID 21653241.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (pdf).
External links
- Joint FAO/WHO Expert Committee on Food Additives evaluation monograph of Acesulfame Potassium
- FDA approval of Acesulfame Potassium
- FDA approval of Acesulfame Potassium as a General Purpose Sweetener in Food
- International Food Information Council article (IFIC) Foundation Everything You Need to Know About Acesulfame Potassium
- Whole Foods Market Health Info Acesulfame K
- Elmhurst College, Illinois Virtual ChemBook Acesulfame K
- Hazardous substances databank entry at the national library of medicine (outdated source)
- Discovery News Sweeteners Linger in Groundwater

