Sodium thiopental
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5.5[1]-26 hours[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.694 |
| Chemical and physical data | |
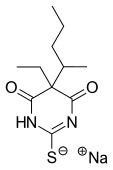
| Formula | C11H17N2NaO2S |
| Molar mass | 264.32 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sodium thiopental, also known as Sodium Penisthol (a trademark of Abbott Laboratories), thiopental, thiopentone, or Trapanal (also a trademark), is a rapid-onset short-acting barbiturate general anesthetic. Sodium thiopental is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic healthcare system.[3] It is also usually the first of three drugs administered during most lethal injections in the United States.
Barbiturates
Barbiturates are a class of drugs that act on the GABAA receptor in the brain and spinal cord. The GABAA receptor is an inhibitory channel that decreases neuronal activity, and barbiturates enhance the inhibitory action of the GABAA receptor. Barbiturates, benzodiazepines, and alcohol all bind to the GABAA receptor. Barbiturates that act on the barbiturate binding site of the GABAA receptor directly gate the chloride ion channel of the GABAA receptor, whereas benzodiazepines acting on the benzodiazepine site on the GABAA receptor increase the opening frequency of the chloride ion channel. This explains why overdoses of barbiturates may be lethal whereas overdoses of benzodiazepines alone are typically not lethal. Another explanation is that barbiturates can activate GABA receptors in the absence of the GABA molecule, whereas benzodiazepines need GABA to be present to have an effect: this may explain the more widespread effects of barbiturates in the central nervous system. Barbiturates have anesthetic, sedative, anxiolytic, anticonvulsant and hypnotic properties. Barbiturates do not have analgesic effects.[4]
Further, barbiturates are relatively non-selective compounds that bind to an entire superfamily of ligand-gated ion channels, of which the GABAA receptor channel is only one of several representatives. This superfamily of ion channels includes the neuronal nACHR channel, the 5HT3R channel, the GlyR channel and others. Surprisingly, while GABAA receptor currents are increased by barbiturates (and other general anesthetics), ligand-gated ion channels that are predominantly permeable for cationic ions are blocked by these compounds. For example, neuronal nACHR channels are blocked by clinically relevant anesthetic concentrations of both sodium thiopental and pentobarbital.[5] Such findings implicate (non-GABA-ergic) ligand-gated ion channels, e.g. the neuronal nAChR channel, in mediating some of the (side) effects of barbiturates.[6]
Uses
Anesthesia
Sodium thiopental is an ultra-short-acting barbiturate and has been used commonly in the induction phase of general anesthesia. Its use has been largely replaced with that of propofol. Following intravenous injection the drug rapidly reaches the brain and causes unconsciousness within 30–45 seconds. At one minute, the drug attains a peak concentration of about 60% of the total dose in the brain. Thereafter, the drug distributes to the rest of the body and in about 5–10 minutes the concentration is low enough in the brain such that consciousness returns.[citation needed]
A normal dose of sodium thiopental (usually 4–6 mg/kg) given to a pregnant woman for operative delivery (caesarian section) rapidly makes her unconscious, but the baby in her uterus remains conscious. However, larger or repeated doses can depress the baby.[citation needed]
Sodium thiopental is not used to maintain anesthesia in surgical procedures because, in infusion, it displays zero-order elimination kinetics, leading to a long period before consciousness is regained. Instead, anesthesia is usually maintained with an inhaled anesthetic (gas) agent. Inhaled anesthetics are eliminated relatively quickly, so that stopping the inhaled anesthetic will allow rapid return of consciousness. Sodium thiopental would have to be given in large amounts to maintain an anesthetic plane, and because of its 11.5–26 hour half-life, consciousness would take a long time to return.[7]
In veterinary medicine, sodium thiopental is used to induce anesthesia in animals. Since it is redistributed to fat, certain breeds of dogs – primarily the sight hounds – can have accelerated recoveries from sodium thiopental due to their lack of body fat and their lean body mass. Similarly,obese animals will have prolonged recoveries. Sodium thiopental is always administered intravenously, as it can be fairly irritating; severe tissue necrosis and sloughing can occur if it is injected incorrectly into the tissue around a vein.
Medically induced coma
In addition to anesthesia induction, sodium thiopental was historically used to induce medical comas.[8] It has now been superseded by drugs such as propofol.
Sodium thiopental has a long Context Sensitive Half Time (CSHT), meaning infusions saturate peripheral compartments (fat, muscle etc.). When the infusion is stopped, the drug redistributes from the peripheral tissues back into the blood, prolonging the effect.
Sodium thiopental also exhibits zero order kinetics at higher doses. The rate of elimination becomes constant.
Patients with brain swelling, causing elevation of the intracranial pressure, either secondary to trauma or following surgery, may benefit from this drug. Sodium thiopental , and the barbiturate class of drugs, decrease neuronal activity and therefore decrease the production of osmotically active metabolites, which in turn decreases swelling. Patients with significant swelling have improved outcomes following the induction of coma. Reportedly, thiopental has been shown to be superior to pentobarbital in reducing intracranial pressure.This phenomena is also termed as Reverse steal Effect.[9]
Euthanasia
Sodium thiopental is used intravenously for the purposes of euthanasia. In both Belgium and the Netherlands, where active euthanesia is allowed by law, the standard protocol recommends sodium thiopental as the ideal agent to induce coma, followed by pancuronium bromide.[10]
Intravenous administration is the most reliable and rapid way to accomplish euthanasia. A coma is first induced by intravenous administration of 20 mg/kg thiopental sodium (Nesdonal) in a small volume (10 ml physiological saline). Then, a triple dose of a non-depolarizing skeletal muscle relaxant is given, such as 20 mg pancuronium bromide (Pavulon) or 20 mg vecuronium bromide (Norcuron). The muscle relaxant should be given intravenously to ensure optimal availability but pancuronium bromide may be administered intramuscularly at an increased dosage level of 40 mg.[10]
Lethal injection
Along with pancuronium bromide and potassium chloride, thiopental is used in 34 states of the U.S. to execute prisoners by lethal injection. A very large dose is given to ensure rapid loss of consciousness. Although death usually occurs within ten minutes of the beginning of the injection process, some have been known to take longer.[11] The use of sodium thiopental in execution protocols was challenged in court after a study in the medical journal The Lancet reported autopsies of executed inmates showed the level of thiopental in their bloodstream was insufficient to cause unconsciousness.
On December 8, 2009, the State of Ohio became the first to use a single dose of sodium thiopental for its capital execution, following the failed use of the standard three-drug cocktail during a recent execution, due to inability to locate suitable veins. Kenneth Biros was executed using the single-drug method.[12]
The state of Washington is now the second state in the U.S. to use the single-dose sodium thiopental injections for death penalty executions. On September 10, 2010, Cal Coburn Brown was executed. His was the first execution in the state to use a single dose, single drug injection. His death was pronounced approximately one and a half minutes after the intravenous administration of five grams of the drug.[13]
Truth serum
Thiopental (Pentothal) is still used in some places as a truth serum to weaken the resolve of the subject and make them more compliant to pressure.[14] The barbiturates as a class decrease higher cortical brain functioning. Some psychiatrists hypothesize that because lying is more complex than telling the truth, suppression of the higher cortical functions may lead to the uncovering of the truth. The drug tends to make subjects loquacious and cooperative with interrogators; however, the reliability of confessions made under thiopental is questionable.[15] Sodium thiopental features as a truth serum in several Hollywood films, in comics and other literature, and even in popular music.[16]
Psychiatry
Psychiatrists have used thiopental to desensitize patients with phobias,[17] and to "facilitate the recall of painful repressed memories."[18] One psychiatrist who worked with thiopental is the Dutch Professor Jan Bastiaans, who used this procedure to help relieve trauma in surviving victims of the Holocaust.[19]
Controversies
Following a shortage that led a court to delay an execution in California, a company spokesman for Hospira, the sole American manufacturer of the drug, objected to the use of thiopental in lethal injection. "Hospira manufactures this product because it improves or saves lives, and the company markets it solely for use as indicated on the product labeling. The drug is not indicated for capital punishment, and Hospira does not support its use in this procedure."[20] On January 21, 2011, the company announced that it would stop production of sodium thiopental from its plant in Italy because it could not guarantee Italian authorities that the drug would not be used in executions. Italy was the only viable place where the company could produce sodium thiopental, leaving the United States without a supplier.[21]
Metabolism
Thiopental rapidly and easily crosses the blood brain barrier as it is a lipophilic molecule. As with all lipid-soluble anaesthetic drugs, the short duration of action of sodium thiopental is due almost entirely to its redistribution away from central circulation towards muscle and fat tissue, due to its very high fat:water partition coefficient (aprx 10), leading to sequestration in fat tissue. Once redistributed, the free fraction in the blood is metabolised in the liver. Sodium thiopental is mainly metabolized to pentobarbital,[22] 5-ethyl-5-(1'-methyl-3'-hydroxybutyl)-2-thiobarbituric acid, and 5-ethyl-5-(1'-methyl-3'-carboxypropyl)-2-thiobarbituric acid.[23]
Dosage
The usual dose range for induction of anesthesia using thiopental is from 3 to 7 mg/kg; however, there are many factors that can alter this. Premedication with sedatives such as benzodiazepines or clonidine will reduce requirements, as do specific disease states and other patient factors. Among patient factors are: age, sex, lean body mass. Specific disease conditions that can alter the dose requirements of thiopentone and for that matter any other intravenous anaesthetic are: hypovolemia, burns, azotemia, hepatic failure, hypoproteinemia, etc.
Side effects
As with nearly all anesthetic drugs, thiopental causes cardiovascular and respiratory depression resulting in hypotension, apnea and airway obstruction. For these reasons, only suitably trained medical personnel should give thiopental in an environment suitably equipped to deal with these effects. Side effects include headache, agitated emergence, prolonged somnolence, and nausea. Intravenous administration of sodium thiopental is followed instantly by an odor and/or taste sensation, sometimes described as being similar to rotting onions, or to garlic. The hangover from the side effects may last up to 36 hours.
Although individual molecules of thiopental contain one sulfur atom, it is not a sulfonamide, and does not show allergic reactions of sulfa/sulpha drugs.
Contraindications
Thiopental should not be given in case of liver disease, Addison's disease, myxedema, severe heart disease, severe hypotension, a severe breathing disorder, or a history of porphyria.[24]
Co-administration of pentoxifylline and thiopental causes death by acute pulmonary edema in rats. This pulmonary edema was not mediated by cardiac failure or by pulmonary hypertension but was due to increased pulmonary vascular permeability.[25]
History
Sodium thiopental was discovered in the early 1930s by Ernest H. Volwiler and Donalee L. Tabern, working for Abbott Laboratories. It was first used in human beings on March 8, 1934, by Dr. Ralph M. Waters[26] in an investigation of its properties, which were short-term anesthesia and surprisingly little analgesia.[27] Three months later,[28] Dr. John S. Lundy started a clinical trial of thiopental at the Mayo Clinic at the request of Abbott.[29] Abbott continued to make the drug until 2004, when it spun off its hospital-products division as Hospira.
Thiopental is famously associated with a number of anesthetic deaths in victims of the attack on Pearl Harbor. These deaths, relatively soon after the substance's discovery, were due to excessive doses given to shocked trauma patients. Evidence has become available through freedom of information legislation and has been reviewed in the British Journal of Anaesthesia.[30] Thiopental anaesthesia was in its early days, but nevertheless 13 of 344 wounded admitted to the Tripler Army Hospital did not survive.
Thiopental is still rarely used as a recreational drug, usually stolen from veterinarians or other legitimate users of the drug; however, more common sedatives such as benzodiazepines are usually preferred as recreational drugs, and abuse of thiopental tends to be uncommon and opportunistic.
Chemistry
Thiopental, 5-ethyl-5-(1-methylbutyl)2-thiobarbituric acid, is synthesized by the alkylation of ethylmalonic ester with 2-bromopentane in the presence of sodium ethoxide. The product ethyl-(1-methylbutyl)malonic ester undergoes heterocyclization with thiourea, using sodium ethoxide as a base.

- E.H. Volwlier, D.L. Tabern, U.S. patent 2,153,729 (1939).
- G.H. Donaldson, W. Bay, U.S. patent 2,876,225 (1959).
See also
References
- ^ Russo H, Brès J, Duboin MP, Roquefeuil B (1995). "Pharmacokinetics of thiopental after single and multiple intravenous doses in critical care patients". Eur. J. Clin. Pharmacol. 49 (1–2): 127–37. doi:10.1007/BF00192371. PMID 8751034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morgan DJ, Blackman GL, Paull JD, Wolf LJ (1981). "Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section". Anesthesiology. 54 (6): 474–80. doi:10.1097/00000542-198106000-00006. PMID 7235275.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. Retrieved 2006-03-12.
{{cite web}}: CS1 maint: year (link) - ^ "Anesthesia and Analgesia". University of Virginia School of Medicine. Retrieved 2007-08-05.
- ^ Weber, M; Motin, L; Gaul, S; Beker, F; Fink, RH; Adams, DJ (2005). "Intravenous anesthetics inhibit nicotinic acetyl-choline receptor-mediated currents and Ca2+ transients in rat intracardiac ganglion neurons". British Journal of Pharmacology. 144 (1): 98–107. doi:10.1038/sj.bjp.0705942. PMC 1575970. PMID 15644873.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Franks, NP; Lieb, WR (23 November 1998). "Which molecular targets are most relevant to general anaesthesia?". Toxicology Letters. 100–101 (1–2): 1–8. doi:10.1016/S0378-4274(98)00158-1. PMID 10049127.
- ^ Morgan DJ, Blackman GL, Paull JD, Wolf LJ (1981). "Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section". Anesthesiology. 54 (6): 474–80. doi:10.1097/00000542-198106000-00006. PMID 7235275.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barbiturate Coma trauma.org
- ^ Pérez-Bárcena J; Barceló B; Homar J; et al. (2005). "[Comparison of the effectiveness of pentobarbital and thiopental in patients with refractory intracranial hypertension. Preliminary report of 20 patients]" (PDF). Neurocirugia (Astur) (in Spanish; Castilian). 16 (1): 5–12, discussion 12–3. PMID 15756405. Retrieved 2008-07-18.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: unrecognized language (link) - ^ a b Royal Dutch Society for the Advancement of Pharmacy (1994). "Administration and Compounding of Euthanasic Agents". The Hague. Retrieved 2008-07-18.
- ^ "Ohio executes inmate with 1-drug lethal injection". AP. December 2001. Retrieved 2009-12-08.
{{cite web}}: CS1 maint: year (link) - ^ Martinez, Edecio (8 December 2009). "Kenneth Biros Execution: Ohio Man First to Die Under 1-Drug Thiopental Sodium Method". CBS News.
- ^ Sullivan, Jennifer (10 September 2010). "Killer on death row 16-1/2 years is executed". The Seattle Times.
- ^ "Truth serum used on 'serial child killers'". Sydney Morning Herald. Reuters. January 12, 2007.
- ^ Anne Bannon; Stevens, Serita Deborah (2007). The Howdunit Book of Poisons (Howdunit). Cincinnati: Writers Digest Books. ISBN 1-58297-456-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Truth Serums". Television Tropes & Idioms. Retrieved 27 July 2012.
- ^ Pearlman, T. (1980). "Behavioral desensitization of phobic anxiety using thiopental sodium". The American Journal of Psychiatry. 137 (12). American Psychiatric Association: 1580–1582. PMID 6108082.
- ^ "Drugged Future?". TIME. February 24, 1958.
- ^ Snelders, Stephen (1998). "The LSD Therapy Career of Jan Bastiaans, M.D". Newsletter of the Multidisciplinary Association for Psychedelic Studies. 8 (1). Multidisciplinary Association for Psychedelic Studies: 18–20.
- ^ McKinley, Jesse (28 September 2010). "Judges Question California's Motivation on Execution". New York Times.
- ^ "U.S. Drug Maker Discontinues Key Death Penalty Drug". Fox News. 21 January 2011.
- ^ WINTERS WD, SPECTOR E, WALLACH DP, SHIDEMAN FE (1955). "Metabolism of thiopental-S35 and thiopental-2-C14 by a rat liver mince and identification of pentobarbital as a major metabolite". J. Pharmacol. Exp. Ther. 114 (3): 343–57. PMID 13243246. Retrieved 2008-07-18.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) [dead link] - ^ Bory C; Chantin C; Boulieu R; et al. (1986). "[Use of thiopental in man. Determination of this drug and its metabolites in plasma and urine by liquid phase chromatography and mass spectrometry]". C. R. Acad. Sci. III, Sci. Vie (in French). 303 (1): 7–12. PMID 3093002.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ "Pentothal (thiopental)". eMedicineHealth. April 12, 2009.
- ^ Pereda J; Gómez-Cambronero L; Alberola A; et al. (2006). "Co-administration of pentoxifylline and thiopental causes death by acute pulmonary oedema in rats". Br. J. Pharmacol. 149 (4): 450–5. doi:10.1038/sj.bjp.0706871. PMC 1978439. PMID 16953192.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ "This Month in Anesthesia History: March". Anesthesia History Association.
- ^ Steinhaus, John E (2001). "The Investigator and His 'Uncompromising Scientific Honesty'". Asa Newsletter. 65 (9). American Society of Anesthesiologists: 7–9.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lundy, John S. (1966). "From this point in time: Some memories of my part in the history of anesthesia". Journal of the American Association of Nurse Anesthetists. 24 (2). American Association of Nurse Anesthetists: 95–102.
- ^ Thatcher, Virginia S. (1953). "Chapter 7: Illegal or Legal?". History of Anesthesia with Emphasis on the Nurse Specialist. J.B. Lippincott. ISBN 0-8240-6525-5.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Bennetts FE (1995). "Thiopentone anaesthesia at Pearl Harbor". Br J Anaesth. 75 (3): 366–8. PMID 7547061. Retrieved 2008-07-18.
{{cite journal}}: Unknown parameter|month=ignored (help)
