Autoinducer-2
| |
| Names | |
|---|---|
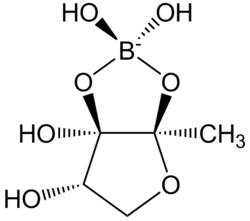
| IUPAC name
(3aS,6S,6aR)-2,2,6,6a-tetrahydroxy-3a-methyltetrahydrofuro[3,2-d][1,3,2]dioxaborolan-2-uide)
| |
| Other names
Dihydroxy[(2S,3R,4S)-2-methyldihydro-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10BO7 | |
| Molar mass | 192.940 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Autoinducer-2 (AI-2), a furanosyl borate diester, is a member of a family of signaling molecules used in quorum sensing.[1] AI-2 is unique in that it is one of only a few known biomolecules incorporating boron. First identified in the marine bacterium Vibrio harveyi, AI-2 is produced and recognized by many Gram-negative and Gram-positive bacteria.[2][3] AI-2 is synthesized by the reaction of 1-deoxy-3-dehydro-D-ribulose with boric acid.[4]
AI-2 is sensed by the Lsr transport cassette and is actively transported into the cell,[clarification needed] where it is phosphorylated by Template:SWL. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the lsr genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the lsr operon. In the presence of glucose, low levels of cAMP/CRP result in almost no lsr operon (lsrABCDFG) expression. Without glucose, cAMP-CRP is needed to stimulate the lsr expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the lsr transcription is de-repressed, enabling even more AI-2 uptake.[5]
Doubts have been expressed regarding AI-2's status as a universal signal. The gene responsible for its production is the widespread luxS; this gene has an important role in the recycling of S-adenosyl-L-methionine, with AI-2 being a metabolic by-product of that process.[6] While it is certainly true that some bacteria respond to AI-2, it is not yet clear that it is always being produced for purposes of signalling.
References
- ^ Cao, Jie-Gang; Meighen, Edward A. (1989). "Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi". Journal of Biological Chemistry. 264 (36): 21670–21676. PMID 2600086.
- ^ Miller, Stephen T.; Xavier, Karina B.; Campagna, Shawn R.; Taga, Michiko E.; Semmelhack, Martin F.; Bassler, Bonnie L.; Hughson, Frederick M. (2004). "Salmonella typhimurium Recognizes a Chemically Distinct Form of the Bacterial Quorum-Sensing Signal AI-2". Molecular Cell. 15 (5): 677–687. doi:10.1016/j.molcel.2004.07.020. PMID 15350213.
- ^ Miller, M. B.; Bassler, B. L. (2001). "Quorum sensing in bacteria". Annual Review of Microbiology. 55: 165–199. doi:10.1146/annurev.micro.55.1.165. PMID 11544353.
- ^ http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/AI2.html
- ^ Wang, Liang; Hashimoto, Yoshifumi; Tsao, Chen-Yu; Valdes, James J.; Bentley, William E. (2005). "Cyclic AMP (cAMP) and cAMP Receptor Protein Influence both Synthesis and Uptake of Extracellular Autoinducer 2 in Escherichia coli". Journal of Bacteriology. 187 (6): 2066–2076. doi:10.1128/JB.187.6.2066-2076.2005. PMC 1064054. PMID 15743955.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1098/rstb.2007.2049, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1098/rstb.2007.2049instead.
