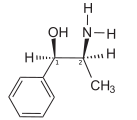
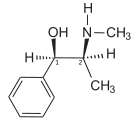
Ephedrine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | oral, IV, IM, SC |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Metabolism | minimal hepatic |
| Elimination half-life | 3–6 hours |
| Excretion | 22-99% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.528 |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ephedrine (/[invalid input: 'ɨ']ˈfɛdrɪn/ or /ˈɛf[invalid input: 'ɨ']driːn/; not to be confused with ephedrone) is a sympathomimetic amine commonly used as a stimulant, appetite suppressant, concentration aid, decongestant, and to treat hypotension associated with anaesthesia.
Ephedrine is similar in molecular structure to the well-known drugs phenylpropanolamine and methamphetamine, as well as to the important neurotransmitter epinephrine (adrenalin). Chemically, it is an alkaloid with a phenethylamine skeleton found in various plants in the genus Ephedra (family Ephedraceae). It works mainly by increasing the activity of norepinephrine (noradrenalin) on adrenergic receptors.[1] It is most usually marketed as the hydrochloride or sulfate salt.
The herb má huáng (麻黄, Ephedra sinica), used in traditional Chinese medicine (TCM), contains ephedrine and pseudoephedrine as its principal active constituents. The same may be true of other herbal products containing extracts from other Ephedra species.
History
Ephedrine in its natural form, known as má huáng (麻黄) in traditional Chinese medicine, has been documented in China since the Han Dynasty (206 BC – 220 AD) as an antiasthmatic and stimulant.[2] The chemical synthesis of ephedrine was first accomplished by Japanese organic chemist Nagai Nagayoshi in 1885. The industrial manufacture of ephedrine in China began in the 1920s, when Merck began marketing and selling the drug as ephetonin. Ephedrine exports between China and the West grew from 4 tonnes to 216 tonnes between 1926 and 1928.[3]
Chemistry

Ephedrine exhibits optical isomerism and has two chiral centres, giving rise to four stereoisomers. By convention the pair of enantiomers with the stereochemistry (1R,2S and 1S,2R) is designated ephedrine, while the pair of enantiomers with the stereochemistry (1R,2R and 1S,2S) is called pseudoephedrine.
Ephedrine is a substituted amphetamine and a structural methamphetamine analogue. It differs from methamphetamine only by the presence of a hydroxyl (OH). Amphetamines, however, are more potent and have additional biological effects.
The isomer which is marketed is (–)-(1R,2S)-ephedrine.[4]
Ephedrine hydrochloride has a melting point of 187−188 °C.[5]
Nomenclature
The dextrorotary (+)- or d- enantiomer is (1S,2R)-Ephedrine, whereas the levorotatory (−)- or l- form is (1R,2S)-Ephedrine.
In the outdated d/l system (+)-Ephedrine is also referred to as l-Ephedrine and (—)-Ephedrine as d-Ephedrine (in the Fisher projection then the phenylring is drawn at bottom).[4][6]
Often the d/l system (with small caps) and the d/l system (with lower-case) are confused. The result is that the levorotary l-Ephedrine is wrongly named l-Ephedrine and the dextrorotary d-Pseudoephedrine (the diastereomer) wrongly d-Pseudoephedrine.
The IUPAC names of the two enantiomers are (1R,2S)- respectively (1S,2R)-2-methylamino-1-phenylpropan-1-ol. A synonym is erythro-Ephedrine.
Agricultural sources
Ephedrine is obtained from the plant Ephedra sinica and other members of the Ephedra genus. Raw materials for the manufacture of ephedrine and traditional Chinese medicines are produced in China on a large scale. As of 2007, companies produced for export US$13 million worth of ephedrine from 30,000 tons of ephedra annually, 10 times the amount that is used in traditional Chinese medicine.[7]
Synthetic sources
Most of the L-ephedrine produced today for official medical use is made synthetically as the extraction and isolation process from Herba Ephedra is tedious and no longer cost effective.[8]
Mechanism of action
Ephedrine is a sympathomimetic amine. The principal mechanism of its action relies on its indirect stimulation of the adrenergic receptor system, which is part of the sympathetic nervous system (SNS), by increasing the activity of noradrenaline at the post-synaptic α- and β-receptors.[1] The presence of direct interactions with α-receptors is unlikely, but still controversial.[9][10][11] L-Ephedrine, and particularly its stereoisomer norpseudoephedrine (which is also present in Catha edulis) has indirect sympathomimetic effects and due to its ability to cross the blood brain barrier, it is a CNS stimulant similar to amphetamines but less pronounced, as it releases noradrenaline and dopamine in the substantia nigra.[12]
The presence of an N-methyl group decreases binding affinities at α-receptors, compared with norephedrine. On the other hand ephedrine binds better than N-methylephedrine, which has an additional methyl group at the N-atom. Also the steric orientation of the hydroxyl group is important for receptor binding and functional activity.[9]
- Compounds with decreasing α-receptor affinity
-
Norephedrine -
Ephedrine -
N-Methylephedrine
Medical use

Indications
In traditional Chinese medicine, má huáng has been used in the treatment of asthma and bronchitis for centuries.[13]
Both ephedrine and pseudoephedrine increase blood pressure and act as bronchodilators, with pseudoephedrine having considerably less effect. [11]
Ephedrine promotes weight loss, specifically fat loss in humans and mice. In mice it is known to stimulate thermogenesis in the brown adipose tissue, however because adult humans have only small amounts of brown fat it is assumed that thermogenesis takes place mostly in the skeletal muscle. Ephedrine also decreases gastric emptying. Methylxanthines like caffeine and theophylline have a synergistic effect with ephedrine with respect to weight loss, and so does aspirin.[citation needed] This led to creation and marketing of compound products.[14] One of them is known as the ECA stack, containing caffeine and aspirin besides ephedrine and is a popular supplement taken by body builders to cut down body fat before a competition.[citation needed]
For many years, the US Coast Guard recommended ephedrine together with an equal 25 mg dose of promethazine to its sailors to combat seasickness. Promethazine manages nausea and ephedrine fights the ensuing drowsiness. Commonly referred to as the Coast Guard cocktail, ephedrine may still be available for prescription for this purpose.[citation needed]
Adverse effects
Adverse drug reactions (ADRs) are more common with systemic administration (e.g. injection or oral administration) compared to topical administration (e.g. nasal instillations). ADRs associated with ephedrine therapy include:[15]
- Cardiovascular: tachycardia, cardiac arrhythmias, angina pectoris, vasoconstriction with hypertension
- Dermatological: flushing, sweating, acne vulgaris
- Gastrointestinal: nausea
- Genitourinary: decreased urination due to vasoconstriction of renal arteries. Also, difficulty urinating is not uncommon, as alpha-agonists such as ephedrine constrict the internal urethral sphincter, mimicking the effects of sympathetic nervous system stimulation.
- Nervous system: restlessness, confusion, insomnia, mild euphoria, mania/hallucinations (rare except in previously existing psychiatric conditions), delusions, formication (may be possible, but lacks documented evidence) paranoia, hostility, panic, agitation
- Respiratory: dyspnea, pulmonary edema
- Miscellaneous: dizziness, headache, tremor, hyperglycemic reactions, dry mouth
The neurotoxicity of l-Ephedrine is disputed. [16]
Contraindications
Ephedrine should not be used in conjunction with certain antidepressants, namely SNRIs (serotonin-norepinephrine re-uptake inhibitors), as this increases the risk of the above symptoms due to excessive serum levels of norepinephrine.
Bupropion is an example of an antidepressant with an amphetamine-like structure similar to ephedrine, and it is known as an NDRI (norepinephrine-dopamine re-uptake inhibitor). It has an action which bears more resemblance to amphetamine than to fluoxetine in that its primary mode of therapeutic action involves norepinephrine and to a lesser degree dopamine, but it also releases some serotonin from presynaptic clefts. It should not be used with ephedrine as it may increase the likelihood of the above side effects.
Ephedrine should be used with caution in patients with inadequate fluid replacement, impaired adrenal function, hypoxia, hypercapnia, acidosis, hypertension, hyperthyroidism, prostatic hypertrophy, diabetes mellitus, cardiovascular disease, during delivery if maternal BP > 130/80 mmHg, and lactation.[17]
Contraindications for the use of ephedrine include: closed angle glaucoma, phaeochromocytoma, asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis), concomitant or recent (previous 14 days) monoamine oxidase inhibitor (MAOI) therapy, general anaesthesia with halogenated hydrocarbons (particularly halothane), tachyarrhythmias or ventricular fibrillation, hypersensitivity to ephedrine or other stimulants.
Ephedrine should not be used at any time during pregnancy unless specifically indicated by a qualified physician and only when other options are unavailable.[17]
Recreational and illicit use
This article needs additional citations for verification. (February 2007) |

Anecdotal reports have suggested that ephedrine helps studying, thinking, or concentrating to a greater extent than caffeine. Some students and some white-collar workers have used ephedrine (or Ephedra-containing herbal supplements) for this purpose, as well as some professional athletes and weightlifters. It is common for many athletes to use stimulants while exercising. Such use of ephedrine has been associated with stimulant dependence, as well as deaths from heatstroke in athletes and circulatory problems such as aortic aneurysm in weightlifters, though these side effects are rare.
As a phenylethylamine, ephedrine has a similar chemical structure to amphetamines and is a methamphetamine analogue having the methamphetamine structure with a hydroxyl group at the β position. Because of ephedrine's structural similarity to methamphetamine it can be used to create methamphetamine using chemical reduction in which ephedrine's hydroxyl group is removed; this has made ephedrine a highly sought-after chemical precursor in the illicit manufacture of methamphetamine. The most popular method for reducing ephedrine to methamphetamine is similar to the Birch reduction, in that it uses anhydrous ammonia and lithium metal in the reaction. The second most popular method uses red phosphorus, iodine, and ephedrine in the reaction.
In E for Ecstasy[18] (a book examining the uses of the drug MDMA in the UK) the writer, activist and Ecstasy advocate Nicholas Saunders highlighted test results showing that certain consignments of the drug also contained ephedrine. Consignments of Ecstasy known as "Strawberry" contained what Saunders described as a "potentially dangerous combination of ketamine, ephedrine and selegiline," as did a consignment of "Sitting Duck" Ecstasy tablets.[19]
Through oxidation, ephedrine can be easily synthesized into methcathinone. Ephedrine is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[20]
Detection of use
Ephedrine may be quantitated in blood, plasma or urine to monitor possible abuse by athletes, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Many commercial immunoassay screening tests directed at the amphetamines cross-react appreciably with ephedrine, but chromatographic techniques can easily distinguish ephedrine from other phenethylamine derivatives. Blood or plasma ephedrine concentrations are typically in the 20-200 µg/L range in persons taking the drug therapeutically, 300-3000 µg/L in abusers or poisoned patients and 3–20 mg/L in cases of acute fatal overdosage. The current WADA limit for ephedrine in an athlete's urine is 10 µg/L.[21][22][23]
Other uses
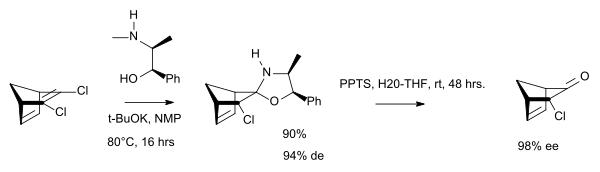
In chemical synthesis, ephedrine is used in bulk quantities to produce chiral auxiliary groups. [24]
Legality
Canada
In January 2002, Health Canada issued a voluntary recall of all ephedrine products containing more than 8 mg per dose, all combinations of ephedrine with other stimulants such as caffeine, and all ephedrine products marketed for weight-loss or bodybuilding indications, citing a serious risk to health.[25] Ephedrine is still sold as an oral nasal decongestant in 8 mg pills, OTC.
USA
In 1997, the FDA proposed a regulation on ephedra (the herb from which ephedrine is obtained), which limited an ephedra dose to 8 mg (of active ephedrine) with no more than 24 mg per day.[26] This proposed rule was withdrawn in part in 2000 because of "concerns regarding the agency's basis for proposing a certain dietary ingredient level and a duration of use limit for these products."[27] In 2004, the FDA created a ban on ephedrine alkaloids that are marketed for reasons other than asthma, colds, allergies, other disease, or traditional Asian use.[28] On April 14, 2005, the U.S. District Court for the District of Utah ruled that the FDA did not have proper evidence that low dosages of ephedrine alkaloids are actually unsafe,[29] but on August 17, 2006, the U.S. Court of Appeals for the Tenth Circuit in Denver upheld the FDA's final rule declaring all dietary supplements containing ephedrine alkaloids adulterated, and therefore illegal for marketing in the United States.[30] Furthermore, ephedrine is banned by NCAA, MLB, NFL, and PGA TOUR.[31] Ephedrine is, however, still legal in many applications outside of dietary supplements. However, purchasing is currently limited and monitored, with specifics varying from state to state.
The House passed the Combat Methamphetamine Epidemic Act of 2005 as an amendment to the renewal of the USA PATRIOT Act. Signed into law by president George W. Bush on March 6, 2006, the act amended the US Code (21 USC 830) concerning the sale of ephedrine-containing products. The federal statute included the following requirements for merchants who sell these products:
- A retrievable record of all purchases identifying the name and address of each party to be kept for two years
- Required verification of proof of identity of all purchasers
- Required protection and disclosure methods in the collection of personal information
- Reports to the Attorney General of any suspicious payments or disappearances of the regulated products
- Non-liquid dose form of regulated product may only be sold in unit dose blister packs
- Regulated products are to be sold behind the counter or in a locked cabinet in such a way as to restrict access
- Daily sales of regulated products not to exceed 3.6 grams without regard to the number of transactions
- Monthly sales not to exceed 9 grams of pseudoephedrine base in regulated products
The law gives similar regulations to mail-order purchases, except the monthly sales limit is only 7.5 grams.
As a pure herb or tea, má huáng, containing ephedrine, is still sold legally in the USA. The law restricts/prohibits its being sold as a dietary supplement(pill) or as an ingredient/additive to other products, like diet pills.
UK
In the UK ephedrine is regulated as a P medicine: it may only be lawfully supplied within a registered pharmacy and while a pharmacist is present. It is not a currently Controlled Drug under the Misuse of Drugs Act. Due to its role in the manufacture of methamphetamine, pure ephedrine is a Category 1 restricted precursor. This makes it illegal to possess, supply or manufacture without a license.
South Africa
In South Africa ephedrine was rescheduled to Schedule 6 on 27 May 2008,[32] which makes the substance legal to possess but available via a prescription only.
Synthesis
Ephedrine can be synthesized from benzaldehyde in a few different ways. According to the first, benzaldehyde is condensed with nitroethane, giving 2-methyl-2-nitro-1-phenylethanol, which is reduced to 2-methyl-2-amino-1-phenylethanol.[33][34] The necessary L-isomer is isolated from the mixture of isomers by crystallization. Methylation of this gives ephedrine.
The second method consists of the fermentation of glucose by yeast carboligase in the presence of benzaldehyde, which during the process turns into phenylacetylcarbinol. This is reduced by hydrogen in the presence of methylamine to give the desired ephedrine.[35][36]
See also
- Amphetamine
- Ephedra
- Halostachine
- Methamphetamine
- Methcathinone
- Phenethylamine
- Pseudoephedrine
- Synephrine
References
- ^ a b Merck Manuals > EPHEDrine Last full review/revision January 2010
- ^ Woodburne O. Levy; Kavita Kalidas (26 February 2010). Norman S. Miller (ed.). Principles of Addictions and the Law: Applications in Forensic, Mental Health, and Medical Practice. Academic Press. pp. 307–308. ISBN 978-0-12-496736-6.
- ^ Frank Dikotter; Lars Peter Laamann (16 April 2004). Narcotic Culture: A History of Drugs in China. University of Chicago Press. p. 199. ISBN 978-0-226-14905-9.
{{cite book}}:|access-date=requires|url=(help) - ^ a b Martindale (1989). Edited by Reynolds JEF (ed.). Martindale: The complete drug reference (29th ed.). London: Pharmaceutical Press. ISBN 0-853690-6.
{{cite book}}:|editor=has generic name (help); Check|isbn=value: length (help) - ^ Budavari S, editor. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals, 12th edition. Whitehouse Station: Merck
- ^ Popat N. Patil, A. Tye and J.B. LaPidus A pharmacological study of the ephedrine isomers JPET May 1965 vol. 148, no. 2, pp. 158-168. Full PDF
- ^ Long, Professor. http://www.chinadialogue.net/article/show/single/en/692-Chinese-medicine-s-great-waste-of-resources
- ^ Chemically Synthesized Ephedrine Put Into Mass Production in China
- ^ a b Guoyi Ma, et al. Pharmacological Effects of Ephedrine Alkaloids on Human {alpha}1- and {alpha}2-Adrenergic Receptor Subtypes J. Pharmacol. Exp. Ther.; nr. 322 pp. 214-221 (july 2007) PDF
- ^ Shigeaki Kobayashi, et al. The Sympathomimetic Actions of l-Ephedrine and d-Pseudoephedrine: Direct Receptor Activation or Norepinephrine Release? Anesth Analg 2003; 97, pp.1239-1245.
- ^ a b Drew, et al. Comparison of the effects of D-(-)-ephedrine and L-(+)-pseudoephedrine on the cardiovascular and respiratory systems in man. Br J Clin Pharmacol. 1978; 6, pp 221-225. PDF
- ^ Munhall AC, Johnson SW. (2006). “Dopamine-mediated actions of ephedrine in the rat substantia nigra.” Brain Res. 1069 (1 ): 96-103. PMID 16386715
- ^ Ford MD, Delaney KA, Ling LJ, Erickson T, editors. Clinical Toxicology. Philadelphia: WB Saunders; 2001. ISBN 0-7216-5485-1 Research Laboratories; 1996. ISBN 0-911910-12-3
- ^ George A. Bray; Claude Bouchard (2004). Handbook of obesity. CRC Press. pp. 494–496. ISBN 978-0-8247-4773-2.
- ^ Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004. ISBN 0-85369-587-3
- ^ Txsci.oxfordjournals (2000).
- ^ a b Mayne Pharma. Ephedrine sulfate injection DBL (Approved Product Information). Melbourne: Mayne Pharma; 2004
- ^ Saunders, N., & Heron, L., (1993) E for Ecstasy (Paperback), N. Saunders, London. (ISBN 0950162884)
- ^ See: [1] for details online.
- ^ Microsoft Word - RedListE2007.doc
- ^ Schier JG, Traub SJ, Hoffman RS, Nelson LS. Ephedrine-induced cardiac ischemia: exposure confirmed with a serum level. Clin. Toxicol. 41: 849-853, 2003.
- ^ WADA. The World Anti-Doping Code, World Anti-Doping Agency, Montreal, Canada, 2010. url
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 542-544.
- ^ Chiral Polycyclic Ketones via Desymmetrization of Dihaloolefins Giuseppe Borsato, Anthony Linden, Ottorino De Lucchi, Vittorio Lucchini, David Wolstenholme, and Alfonso Zambon J. Org. Chem.; 2007; 72(11) pp 4272 - 4275; (Note) doi:10.1021/jo070222g
- ^ "Health Canada requests recall of certain products containing Ephedra/ephedrine". Health Canada. January 9, 2002. Archived from the original on February 6, 2007. Retrieved July 7, 2009.
- ^ Federal Register: June 4, 1997 (Volume 62, Number 107): Dietary Supplements Containing Ephedrine Alkaloids; Proposed Rule
- ^ Federal Register: April 3, 2000 (Volume 65, Number 64): Dietary Supplements Containing Ephedrine Alkaloids; Withdrawal in Part
- ^ Federal Register: February 11, 2004 (Volume 69, Number 28): Final Rule Declaring Dietary Supplements Containing Ephedrine Alkaloids Adulterated Because They Present an Unreasonable Risk; Final Rule
- ^ [2]
- ^ [3]
- ^ http://www.drugfreesport.com/drug-resources/faq.asp
- ^ http://www.doh.gov.za/docs/pr/2008/pr0527.html
- ^ Spath, K. Gohring, Monatsh. Chem., 41, 319 (1920)
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01377a032, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01377a032instead. - ^ W. Klawehn, G. Hilderbrandt, U.S. patent 1,956,950 (1934)
- ^ Merck Chem. Fab. E, DE 469782 (1926)