Lipase

Lipase is an enzyme that catalyzes the breakdown or hydrolysis of fats (lipids).[1] Lipases are a subclass of the esterases.
Lipases perform essential roles in the digestion, transport and processing of dietary lipids (e.g. triglycerides, fats, oils) in most, if not all, living organisms. Genes encoding lipases are even present in certain viruses.[2][3]
Most lipases act at a specific position on the glycerol backbone of lipid substrate (A1, A2 or A3)(small intestine). For example, human pancreatic lipase (HPL),[4] which is the main enzyme that breaks down dietary fats in the human digestive system, converts triglyceride substrates found in ingested oils to monoglycerides and two fatty acids.
Several other types of lipase activities exist in nature, such as phospholipases [5] and sphingomyelinases,[6] however these are usually treated separately from "conventional" lipases.
Some lipases are expressed secreted by pathogenic organisms during the infection. In particular, Candida albicans has a large number of different lipases, possibly reflecting broad lipolytic activity, which may contribute to the persistence and virulence of C. albicans in human tissue.[7]
Structure and catalytic mechanism
Although a diverse array of genetically distinct lipase enzymes are found in nature, and represent several types of protein folds and catalytic mechanisms, most are built on an alpha/beta hydrolase fold[8][9][10] (see image[11]) and employ a chymotrypsin-like hydrolysis mechanism involving a serine nucleophile, an acid residue (usually aspartic acid), and a histidine.[12][13]
Physiological distribution
Lipases are involved in diverse biological processes ranging from routine metabolism of dietary triglycerides to cell signaling[14] and inflammation.[15] Thus, some lipase activities are confined to specific compartments within cells while others work in extracellular spaces.
- In the example of lysosomal lipase, the enzyme is confined within an organelle called the lysosome.
- Other lipase enzymes, such as pancreatic lipases, are secreted into extracellular spaces where they serve to process dietary lipids into more simple forms that can be more easily absorbed and transported throughout the body.
- Fungi and bacteria may secrete lipases to facilitate nutrient absorption from the external medium (or in examples of pathogenic microbes, to promote invasion of a new host).
- Certain wasp and bee venoms contain phospholipases that enhance the "biological payload" of injury and inflammation delivered by a sting.
- As biological membranes are integral to living cells and are largely composed of phospholipids, lipases play important roles in cell biology.
- Malassezia globosa, a fungus that is thought to be the cause of human dandruff, uses lipase to break down sebum into oleic acid and increase skin cell production, causing dandruff.[16]
Human lipases
The main lipases of the human digestive system are human pancreatic lipase (HPL) and pancreatic lipase related protein 2 (PLRP2), which are secreted by the pancreas. Humans also have several other related enzymes, including hepatic lipase (HL), endothelial lipase, and lipoprotein lipase. Not all of these lipases function in the gut (see table).
| Name | Gene | Location | Description | Disorder |
| bile salt dependent lipase | ? | pancreas, breast milk | aids in the digestion of fats | |
| pancreatic lipase | PNLIP | digestive juice | In order to exhibit optimal enzyme activity in the gut lumen, HPL requires another protein, colipase, which is also secreted by the pancreas.[17] | |
| lysosomal lipase | LIPA | interior space of organelle: lysosome | Also referred to as lysosomal acid lipase (LAL or LIPA) or acid cholesteryl ester hydrolase | Cholesteryl ester storage disease (CESD) and Wolman disease are both caused by mutations in the gene encoding lysosomal lipase.[18] |
| hepatic lipase | LIPC | endothelium | Hepatic lipase acts on the remaining lipids carried on lipoproteins in the blood to regenerate LDL (low density lipoprotein). | - |
| lipoprotein lipase | LPL or "LIPD" | endothelium | Lipoprotein lipase functions in the blood to act on triacylglycerides carried on VLDL (very low density lipoprotein) so that cells can take up the freed fatty acids. | Lipoprotein lipase deficiency is caused by mutations in the gene encoding lipoprotein lipase.[19][20] |
| hormone-sensitive lipase | LIPE | intracellular | - | - |
| gastric lipase | LIPF | digestive juice | Functions in the infant at a near-neutral pH to aid in the digestion of lipids | - |
| endothelial lipase | LIPG | endothelium | - | - |
| pancreatic lipase related protein 2 | PNLIPRP2 or "PLRP2" - | digestive juice | - | - |
| pancreatic lipase related protein 1 | PNLIPRP1 or "PLRP1" | digestive juice | Pancreatic lipase related protein 1 is very similar to PLRP2 and HPL by amino acid sequence (all three genes probably arose via gene duplication of a single ancestral pancreatic lipase gene). However, PLRP1 is devoid of detectable lipase activity and its function remains unknown, even though it is conserved in other mammals.[21][22] | - |
| lingual lipase | ? | digestive juice | Active at gastric pH levels. Optimum pH is about 3.5-6. Screted by the Parotid and Ebner's glands at the back of the tongue. | - |
Other lipases include LIPH, LIPI, LIPJ, LIPK, LIPM, LIPN, MGLL, DAGLA, DAGLB, and CEL.
There also are a diverse array of phospholipases, but these are not always classified with the other lipases.
Industrial uses
Lipases serve important roles in human practices as ancient as yogurt and cheese fermentation. However, lipases are also being exploited as cheap and versatile catalysts to degrade lipids in more modern applications. For instance, a biotechnology company has brought recombinant lipase enzymes to market for use in applications such as baking, laundry detergents and even as biocatalysts[23] in alternative energy strategies to convert vegetable oil into fuel.[24][25]High enzyme activity lipase can replace traditional catalyst in processing biodiesel, this enzyme is more environmental and safe. Industrial applicaton of lipases requires process intensification for continuous processing using tools like continuous flow microreactors at small scale[26] [27]
Source
Lipases are generally animal sourced, but can also be sourced microbially. Serum lipase values in the human body range normally from 0-160 U/L.[28]
Additional images
-
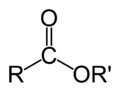
General formula of a carboxylate ester
See also
- Alpha toxin
- Lysosomal acid lipase deficiency
- Peripheral membrane proteins
- Phospholipase A
- Phospholipase C
- Triglyceride lipase
References
- ^ Svendsen A (2000). "Lipase protein engineering". Biochim Biophys Acta. 1543 (2): 223–228. doi:10.1016/S0167-4838(00)00239-9. PMID 11150608.
- ^ Afonso C, Tulman E, Lu Z, Oma E, Kutish G, Rock D (1999). "The Genome of Melanoplus sanguinipes Entomologists". J Virol. 73 (1): 533–52. PMC 103860. PMID 9847359.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Girod A, Wobus C, Zádori Z, Ried M, Leike K, Tijssen P, Kleinschmidt J, Hallek M (2002). "The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity". J Gen Virol. 83 (Pt 5): 973–8. PMID 11961250.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Winkler FK, D'Arcy A, and W Hunziker (1990). "Structure of human pancreatic lipase". Nature. 343 (6260): 771–774. doi:10.1038/343771a0. PMID 2106079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Diaz, B.L., and J. P. Arm. (2003). "Phospholipase A(2)". Prostaglandins Leukot Essent Fatty Acids. 2–3 (2–3): 87–97. doi:10.1016/S0952-3278(03)00069-3. PMID 12895591.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goñi F, Alonso A (2002). "Sphingomyelinases: enzymology and membrane activity". FEBS Lett. 531 (1): 38–46. doi:10.1016/S0014-5793(02)03482-8. PMID 12401200.
- ^ Hube B, Stehr F, Bossenz M, Mazur A, Kretschmar M, Schafer W (2000). "Secreted lipases of Candida albicans: cloning, characterisation and expression analysis of a new gene family with at least ten members". Arch. Microbiol. 174 (5): 362–374. doi:10.1007/s002030000218. PMID 11131027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Winkler FK, D'Arcy A, and W Hunziker (1990). "Structure of human boob pancreatic lipase". Nature. 343 (6260): 771–774. doi:10.1038/343771a0. PMID 2106079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schrag J, Cygler M (1997). "Lipases and alpha/beta hydrolase fold". Methods Enzymol. Methods in Enzymology. 284: 85–107. doi:10.1016/S0076-6879(97)84006-2. ISBN 978-0-12-182185-2. PMID 9379946.
- ^ Egmond, M. R., and C. J. van Bemmel (1997). "Impact of Structural Information on Understanding of Lipolytic Function". Methods Enzymol. Methods in Enzymology. 284: 119–129. doi:10.1016/S0076-6879(97)84008-6. ISBN 978-0-12-182185-2. PMID 9379930.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Withers-Martinez C, Carriere F, Verger R, Bourgeois D, and C Cambillau (1996). "A pancreatic lipase with a phospholipase A1 activity: crystal structure of a chimeric pancreatic lipase-related protein 2 from guinea pig". Structure. 4 (11): 1363–74. doi:10.1016/S0969-2126(96)00143-8. PMID 8939760.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brady, L., A. M. Brzozowski, Z. S. Derewenda, E. Dodson, G. Dodson, S. Tolley, J. P. Turkenburg, L. Christiansen, B. Huge-Jensen, L. Norskov, and; et al. (1990). "A serine protease triad forms the catalytic centre of a triacylglycerol lipase". Nature. 343 (6260): 767–70. doi:10.1038/343767a0. PMID 2304552.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lowe ME (1992). "The catalytic site residues and interfacial binding of human pancreatic lipase". J Biol Chem. 267 (24): 17069–73. PMID 1512245.
- ^ Spiegel S, Foster D, and R Kolesnick (1996). "Signal transduction through lipid second messengers". Curr Opin Cell Biol. 8 (2): 159–67. doi:10.1016/S0955-0674(96)80061-5. PMID 8791422.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tjoelker LW, Eberhardt C, Unger J, Trong HL, Zimmerman GA, McIntyre TM, Stafforini DM, Prescott SM, and PW Gray (1995). "Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad". J Biol Chem. 270 (43): 25481–7. doi:10.1074/jbc.270.43.25481. PMID 7592717.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Genetic Code of Dandruff Cracked - BBC News
- ^ Lowe ME (2002). "The triglyceride lipases of the pancreas". J Lipid Res. 43 (12): 2007–16. doi:10.1194/jlr.R200012-JLR200. PMID 12454260.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Omim - Wolman Disease
- ^ Familial lipoprotein lipase deficiency - Genetics Home Reference
- ^ Gilbert B, Rouis M, Griglio S, de Lumley L, Laplaud P (2001). "Lipoprotein lipase (LPL) deficiency: a new patient homozygote for the preponderant mutation Gly188Glu in the human LPL gene and review of reported mutations: 75 % are clustered in exons 5 and 6". Ann Genet. 44 (1): 25–32. doi:10.1016/S0003-3995(01)01037-1. PMID 11334614.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crenon I, Foglizzo E, Kerfelec B, Verine A, Pignol D, Hermoso J, Bonicel J, Chapus C (1998). "Pancreatic lipase-related protein type I: a specialized lipase or an inactive enzyme". Protein Eng. 11 (2): 135–42. doi:10.1093/protein/11.2.135. PMID 9605548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ De Caro J, Carriere F, Barboni P, Giller T, Verger R, De Caro A (1998). "Pancreatic lipase-related protein 1 (PLRP1) is present in the pancreatic juice of several species". Biochim Biophys Acta. 1387 (1–2): 331–41. doi:10.1016/S0167-4838(98)00143-5. PMID 9748646.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Guo Z, Xu X (2005). "New opportunity for enzymatic modification of fats and oils with industrial potentials". Org Biomol Chem. 3 (14): 2615–9. doi:10.1039/b506763d. PMID 15999195.
- ^ Gupta R, Gupta N, Rathi P (2004). "Bacterial lipases: an overview of production, purification and biochemical properties". Appl Microbiol Biotechnol. 64 (6): 763–81. doi:10.1007/s00253-004-1568-8. PMID 14966663.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ban K, Kaieda M, Matsumoto T, Kondo A, Fukuda H (2001). "Whole cell biocatalyst for biodiesel fuel production utilizing Rhizopus oryzae cells immobilized within biomass support particles". Biochem Eng J. 8 (1): 39–43. doi:10.1016/S1369-703X(00)00133-9. PMID 11356369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhangale, Atul. "Enzyme-Catalyzed Polymerization of End-Functionalized Polymers in a Microreactor". Macromolecules.
- ^ Bhangale, Atul. "Continuous Flow Enzyme-Catalyzed Polymerization in a Microreactor". JACS.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol 2002; 97:1309–1318".
{{cite web}}:|first=missing|last=(help)
25. Gulzar, Bio-degradation of hydrocarbons using different bacterial and fungal species. Published in international conference on biotechnology and neurosciences. CUSAT (cochin university of science and technology), 2004
External links
- Lipase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Selective Inhibitors of Monoacylglycerol Lipase as a Treatment for Neurological Disorders 2004-637
- UMich Orientation of Proteins in Membranes families/superfamily-90 - Phospholipases A2
- UMich Orientation of Proteins in Membranes families/superfamily-29 - Outer membrane phospholipase A
- UMich Orientation of Proteins in Membranes families/superfamily-134 - Cytosolic phospholipase A2 and patatin
- UMich Orientation of Proteins in Membranes families/superfamily-126 - Bacterial and mammalian phospholipases C
- UMich Orientation of Proteins in Membranes families/superfamily-88 - α-toxin (a bacterial phospholipase C)