Naproxen
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Aleve |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681029 |
| License data | |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% (oral) |
| Protein binding | 99% |
| Metabolism | Hepatic (to 6-desmethylnaproxen) |
| Elimination half-life | 12–24 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.747 |
| Chemical and physical data | |
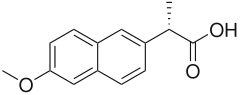
| Formula | C14H14O3 |
| Molar mass | 230.259 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Naproxen sodium (INN) /nəˈprɒksən/ is a nonsteroidal anti-inflammatory drug (NSAID) and is commonly used for relief of a wide variety of pain, fever, inflammations, and stiffness.
Medical uses
Naproxen is commonly used for the reduction of pain, fever, inflammation and stiffness caused by conditions including migraine, osteoarthritis, kidney stones, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, menstrual cramps, tendinitis and bursitis. It is also used for the treatment of primary dysmenorrhea.[2]
Diagnostics
Naproxen has been utilized to differentiate between infectious fevers and those with neoplastic or connective tissue disease related fevers.[3][4]
Adverse effects
COX-2 selective and nonselective NSAIDs have been linked to increases in the number of serious and potentially fatal cardiovascular events, such as myocardial infarctions and strokes. Naproxen is however associated with the smallest overall cardiovascular risks.[5][6] The drug had roughly 50% of the associated risk of stroke as compared with ibuprofen and was also associated with a reduced number of myocardial infarctions as compared to control groups.[5] As with other non-COX-2 selective NSAIDs, naproxen can cause gastrointestinal problems, such as heartburn, constipation, diarrhea, ulcers and stomach bleeding.[7] Persons with a history of ulcers or inflammatory bowel disease should consult a doctor before taking naproxen.
It was found that high-dose naproxen induced near-complete suppression of platelet thromboxane throughout the dosing interval and appeared not to increase cardiovascular disease (CVD) risk, whereas other high-dose NSAID regimens had only transient effects on platelet COX-1 and were associated "with a small but definite vascular hazard". Conversely, naproxen was associated with higher rates of upper gastrointestinal bleeding complications in comparison to other NSAIDs.[6]
NSAID painkillers, such as naproxen, may interfere with and reduce the efficacy of SSRI antidepressants.[8][9]
Mechanism of action
Naproxen works by inhibiting both the COX-1 and COX-2 enzymes.[10][11][12][13][14]
Compound information
Naproxen is a member of the 2-arylpropionic acid (profen) family of NSAIDs. The free acid is an odorless, white to off-white, crystalline substance. It is lipid-soluble and practically insoluble in water. It has a melting point of 152–155 °C.
Synthesis
Naproxen has been industrially produced by Syntex as follows:[15]
Other synthetic routes have also been discussed.[15]
Marketing and trade names
Naproxen and naproxen sodium are marketed under various trade names, including: Aleve, Anaprox, Antalgin, Apranax, Feminax Ultra, Flanax, Inza, Midol Extended Relief, Nalgesin, Naposin, Naprelan, Naprogesic, Naprosyn, Narocin, Proxen, Soproxen, Synflex and Xenobid.
Naproxen was originally marketed as the prescription drug Naprosyn by Syntex in 1976, and naproxen sodium was first marketed under the trade name Anaprox in 1980. It remains a prescription-only drug in much of the world. In the United States, the Food and Drug Administration (FDA) approved its use as an over-the-counter (OTC) drug in 1994; OTC preparations in the U.S. are mainly marketed by Bayer HealthCare under the trade name Aleve and generic store brand formulations in 220 mg tablets. In Australia, packets of 275 mg tablets of naproxen sodium are Schedule 2 pharmacy medicines, with a maximum daily dose of five tablets or 1375 mg. In the United Kingdom, 250-mg tablets of naproxen were approved for OTC sale under the brand name Feminax Ultra in 2008, for the treatment of primary dysmenorrhoea in women aged 15 to 50.[16] In the Netherlands, 220mg and 275mg tablets are available OTC in drugstores, 550mg is OTC only at pharmacists. Aleve became available over-the-counter in most provinces in Canada on 14 July 2009, but not British Columbia, Quebec or Newfoundland and Labrador;[17] it became available OTC in British Columbia in late January 2010.[18]
Research
Naproxen may have anti-viral activity against influenza. Specifically, it blocks the RNA-binding groove of the nucleoprotein of the virus, thereby preventing formation of the ribonucleoprotein complex, thus taking the vital nucleoproteins out of circulation.[19][20]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ French L (2005). "Dysmenorrhea" (PDF). Am Fam Physician. 71 (2): 285–91. PMID 15686299.
- ^ Dy, Emmanuel Edwin R.; et al. (1996). "The Naproxen Test as a Diagnostic Tool in the Causative Differentiation of Fever" (PDF). Philipp J Intern Med. 34 (6): 235–239.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Zell JA, Chang JC (2005). "Neoplastic fever: a neglected paraneoplastic syndrome". Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 13 (11): 870–7. doi:10.1007/s00520-005-0825-4. PMID 15864658.
- ^ a b Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P. (2011). "Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis". BMJ. 342: c7086. doi:10.1136/bmj.c7086. PMC 3019238. PMID 21224324. c7086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Coxib and traditional NSAID Trialists' (CNT) Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C (2013). "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials". Lancet. 382 (9894): 769–79. doi:10.1016/S0140-6736(13)60900-9. PMID 23726390.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Naproxen. PubMed Health.
- ^ Tudor, Amy (2011-04-20) Why Painkillers Interfere with Anti-depressants. Healthcentral.com. Retrieved on 2013-09-20.
- ^ Warner-Schmidt JL (2011). "Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans". Proc. Natl. Acad. Sci. U.S.A. 108 (22): 9262–7. doi:10.1073/pnas.1104836108. PMC 3107316. PMID 21518864.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ (2010). "Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen". The Journal of biological chemistry. 285 (45): 34950–9. doi:10.1074/jbc.M110.162982. PMC 2966109. PMID 20810665.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Hinz B, Cheremina O, Besz D, Zlotnick S, Brune K. (2008). "Impact of naproxen sodium at over-the-counter doses on cyclooxygenase isoforms in human volunteers". International journal of clinical pharmacology and therapeutics. 46 (4): 180–6. doi:10.5414/CPP46180. PMID 18397691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Van Hecken A, Schwartz JI, Depré M, De Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, De Schepper PJ (2000). "Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers". Journal of clinical pharmacology. 40 (10): 1109–20. PMID 11028250.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gross GJ, Moore J. (2004). "Effect of COX-1/COX-2 inhibition versus selective COX-2 inhibition on coronary vasodilator responses to arachidonic acid and acetylcholine". Pharmacology. 71 (3): 135–42. doi:10.1159/000077447. PMID 15161995.
- ^ Hawkey CJ (2001). "COX-1 and COX-2 inhibitors". Best practice & research. Clinical gastroenterology. 15 (5): 801–20. doi:10.1053/bega.2001.0236. PMID 11566042.
- ^ a b Peter J. Harrington and Eric Lodewijk (1997). "Twenty Years of Naproxen Technology". Org. Process Res. Dev. 1 (1): 72–76. doi:10.1021/op960009e.
- ^ "Medicines regulator approves availability of a new OTC medicine for period pain" (PDF) (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). 1 April 2008.
- ^ "ALEVE – Welcome to Canada, Eh!" (PDF) (Press release). Bayer Health Care. 14 July 2009. Retrieved 24 March 2012.
- ^ "ALEVE® – Helping British Columbians with Joint and Arthritis Pain Get Back to Doing the Activities They Love". newswire.ca. 28 January 2010.
- ^ reliever shows anti-viral activity against flu. Eurekalert.org (2013-03-21). Retrieved on 2013-09-20.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/AAC.02335-12, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/AAC.02335-12instead.
External links
- Aleve U.S.
- Aleve Canada
- CID 1302 from PubChem
- EINECS number 244-838-7
- MedlinePlus Information on naproxen
- FDA Statement on Naproxen, released 20 December 2004
- Alzheimer's Disease Anti-Inflammatory Prevention Trial
- Forbes article (expressing the point of view that the risk of heart attack or stroke was overstated)
- Which NSAID for Heart Disease Patients? – Medscape
- U.S. National Library of Medicine: Drug Information Portal – Naproxen
- Aleve Daily Med
- Naproxen bound to proteins in the PDB
- Use of naproxen in the Treatment of RSD
- [1] (Chemistry blog describing the extraction and testing of enantiomeric purity of naproxen sodium purchased at Dollar Tree)

