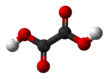
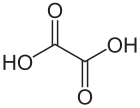
Oxalic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
ethanedioic acid
| |||
| Other names
oxalic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 385686 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.123 | ||
| EC Number |
| ||
| 2208 | |||
| KEGG | |||
| MeSH | Oxalic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3261 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H2O4 | |||
| Molar mass | 90.034 g·mol−1 (anhydrous) 126.07 g mol−1 (dihydrate) | ||
| Appearance | White crystals | ||
| Density | 1.90 g cm−3 (anhydrous) 1.653g cm−3 (dihydrate) | ||
| Melting point | 102 °C (216 °F; 375 K) | ||
| 14.3 g/100ml (25 °C) | |||
| Solubility | 23.7 g/100ml (15 °C) in ethanol 1.4 g/100ml (15 °C) in diethyl ether [1] | ||
| Acidity (pKa) | 1.25, 4.14[2] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 166 °C (331 °F; 439 K) | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxalic acid is an organic compound with the formula H2C2O4. It is a colorless crystalline solid that dissolves in water to give colorless solutions. It is classified as a dicarboxylic acid. In terms of acid strength, it is much stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate (C2O42−), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula H2C2O4·2H2O. Oral consumption of oxalic acid in excess or prolonged skin contact can be dangerous.
Preparation
Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. A variety of precursors can be used including glycolic acid and ethylene glycol.[3] A newer method entails oxidative carbonylation of alcohols to give the diesters of oxalic acid:
- 4 ROH + 4 CO + O2 → 2 (CO2R)2 + 2 H2O
These diesters are subsequently hydrolyzed to oxalic acid. Approximately 120,000 metric tons are produced annually.[4]
Historically oxalic acid was obtained exclusively by using caustics, such as sodium or potassium hydroxide, on sawdust.[5]
Laboratory methods
Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst.[6]
The hydrated solid can be dehydrated with heat or by azeotropic distillation.[7]
Of historical interest, Wöhler prepared oxalic acid by hydrolysis of cyanogen in 1824. This experiment may represent the first synthesis of a natural product.[4]
Structure
Anhydrous oxalic acid exists as two polymorphs; in one the hydrogen-bonding results in a chain-like structure whereas the hydrogen bonding pattern in the other form defines a sheet-like structure.[8] Because the anhydrous material is both acidic and hydrophilic (water seeking), it is used in esterifications.
Reactions
Oxalic acid is a relatively strong acid, despite being a carboxylic acid:
- C2O4H2 → C2O4H− + H+; pKa = 1.27
- C2O4H− → C2O42− + H+; pKa = 4.27
Oxalic acid undergoes many of the reactions characteristic of other carboxylic acids. It forms esters such as dimethyl oxalate (m.p. 52.5–53.5 °C).[9] It forms an acid chloride called oxalyl chloride.
Oxalate, the conjugate base of oxalic acid, is an excellent ligand for metal ions, e.g. the drug oxaliplatin.
Oxalic acid and oxalates can be oxidized by permanganate in an autocatalytic reaction.[10]
Occurrence
Biosynthesis
At least two pathways exist for the enzyme-mediated formation of oxalate. In one pathway, oxaloacetate, a component of the Krebs citric acid cycle, is hydrolyzed to oxalate and acetic acid by the enzyme oxaloacetase:[11]
- [O2CC(O)CH2CO2]2− + H2O → C2O42− + CH3CO2−
It also arises from the dehydrogenation of glycolic acid, which is produced by the metabolism of ethylene glycol.
Occurrence in foods and plants
Calcium oxalate is the most common component of kidney stones. Early investigators isolated oxalic acid from wood-sorrel (Oxalis). Members of the spinach family are high in oxalates, as is sorrel.[12] Rhubarb leaves contain about 0.5% oxalic acid and jack-in-the-pulpit (Arisaema triphyllum) contains calcium oxalate crystals. Bacteria produce oxalates from oxidation of carbohydrates.[4]
Other
Oxidized bitumen or bitumen exposed to gamma rays also contains oxalic acid among its degradation products. Oxalic acid may increase the leaching of radionuclides conditioned in bitumen for radioactive waste disposal.[citation needed]
Biochemistry
The conjugate base of oxalic acid (oxalate) is a competitive inhibitor of the lactate dehydrogenase(LDH) enzyme.[13] LDH catalyses the conversion of pyruvate to lactic acid (End product of the Fermentation (Anaerobic) Process) oxidising the coenzyme NADH to NAD+ and H+ concurrently. Restoring NAD+ levels are essential to the continuation of anaerobic energy metabolism through glycolysis. As cancer cells preferentially use anaerobic metabolism (see Warburg effect) inhibition of LDH has been shown to inhibit tumor formation and growth,[14] thus is an interesting potential course of cancer treatment.
Applications
About 25% of produced oxalic acid is used as a mordant in dyeing processes. It is used in bleaches, especially for pulpwood. It is also used in baking powder.[4]
Cleaning
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent), e.g. Bar Keepers Friend is an example of a household cleaner containing oxalic acid. Its utility in rust removal agents is due to its forming a stable, water soluble salt with ferric iron, ferrioxalate ion.
Extractive metallurgy
Oxalic acid is an important reagent in lanthanide chemistry. Hydrated lanthanide oxalates form readily in strongly acidic solutions in a densely crystalline, easily filtered form, largely free of contamination by nonlanthanide elements. Thermal decomposition of these oxalate gives the oxides, which is the most commonly marketed form of these elements.
Niche uses
Vaporized oxalic acid, or a 3.2% solution of oxalic acid in sugar syrup, is used by some beekeepers as a miticide against the parasitic varroa mite.
Oxalic acid is rubbed onto completed marble sculptures to seal the surface and introduce a shine. Oxalic acid is also used to clean iron and manganese deposits from quartz crystals.[15][16]
Content in food items
This table was originally published in Agriculture Handbook No. 8-11, Vegetables and Vegetable Products, 1984.[17]
| Vegetable | Oxalic acid (g/100 g) |
|---|---|
| Amaranth | 1.09 |
| Asparagus | 0.13 |
| Beans, snap | 0.36 |
| Beet leaves | 0.61 |
| Broccoli | 0.19 |
| Brussels sprouts | 0.36 |
| Cabbage | 0.10 |
| Carrot | 0.50 |
| Cassava | 1.26 |
| Cauliflower | 0.15 |
| Celery | 0.19 |
| Chicory | 0.21 |
| Chives | 1.48 |
| Collards | 0.45 |
| Coriander | 0.01 |
| Corn, sweet | 0.01 |
| Cucumbers | 0.02 |
| Eggplant | 0.19 |
| Endive | 0.11 |
| Garlic | 0.36 |
| Kale | 0.02 |
| Lettuce | 0.33 |
| Okra | 0.05 |
| Onion | 0.05 |
| Parsley | 1.70 |
| Parsnip | 0.04 |
| Pea | 0.05 |
| Bell pepper | 0.04 |
| Potato | 0.05 |
| Purslane | 1.31 |
| Radish | 0.48 |
| Rutabaga | 0.03 |
| Spinach | 0.97 |
| Squash | 0.02 |
| Sweet potato | 0.24 |
| Tomato | 0.05 |
| Turnip | 0.21 |
| Turnip greens | 0.05 |
| Watercress | 0.31 |
Toxicity and safety
Oxalic acid has toxic effects through contact and if ingested; manufacturers provide details in Material Safety Data Sheets (MSDS). It is not identified as mutagenic or carcinogenic; there is a possible risk of congenital malformation in the fetus; may be harmful if inhaled, and is extremely destructive to tissue of mucous membranes and upper respiratory tract; harmful if swallowed; harmful to and destructive of tissue and causes burns if absorbed through the skin or is in contact with the eyes. Symptoms and effects include a burning sensation, cough, wheezing, laryngitis, shortness of breath, spasm, inflammation and edema of the larynx, inflammation and edema of the bronchi, pneumonitis, pulmonary edema.[18]
In humans, ingested oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg.[19][dead link] It has been reported that the lethal oral dose is 15 to 30 grams.[20]
The toxicity of oxalic acid is due to kidney failure caused by precipitation of solid calcium oxalate,[21] the main component of kidney stones. Oxalic acid can also cause joint pain due to the formation of similar precipitates in the joints. Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.
References
- ^ Radiant Agro Chem. "Oxalic Acid MSDS".
- ^ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ^ Yonemitsu Eiichi, Isshiki Tomiya, Suzuki Tsuyoshi, Yashima Yukio, Process for the production of oxalic acid, US 3678107
- ^ a b c d Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a18_247.
- ^ Von Wagner, Rudolf (1897). Manual of chemical technology. New York: D. Appleton & Co. p. 499.
- ^ Practical Organic Chemistry by Julius B. Cohen, 1930 ed. preparation #42
- ^ Clarke H. T.;. Davis, A. W. (1941). "Oxalic acid (anhydrous)". Organic Syntheses: 421
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1. - ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Bowden, E. (1943). "Methyl oxalate". Organic Syntheses: 414; Collected Volumes, vol. 2.
- ^ Kovacs K.A., Grof P., Burai L., Riedel M. (2004). "Revising the mechanism of the permanganate/oxalate reaction". J. Phys. Chem. A. 108 (50): 11026–11031. doi:10.1021/jp047061u.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Martin V. Dutton, Christine S. Evans "Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment" Canadian Journal of Microbiology, 1996, vol. 42, p. 881-895. doi:10.1139/m96-114.
- ^ Rombauer, Rombauer Becker, and Becker (1931/1997). Joy of Cooking, p.415. ISBN 0-684-81870-1.
- ^ Novoa, William (1958). "Lactic Dehydrogenase V. inhibiton by Oxamate and Oxalate". Journal of Biological Chemistry.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Le, Anne (14 December 2009). "Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression". Proceedings of the National Academy of Sciences.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ [1]
- ^ [2]
- ^ http://www.ars.usda.gov/Services/docs.htm?docid=9444
- ^ Sigma-Aldrich: Oxalic acid dihydrate Material Safety Data Sheet (MSDS)
- ^ Safety Officer in Physical Chemistry (August 13, 2005). "Safety (MSDS) data for oxalic acid dihydrate". Oxford University. Retrieved December 30, 2009.
- ^ CDC: Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs), Oxalic acid, May 1994
- ^ EMEA Committee for veterinary medicinal products, oxalic acid summary report, December 2003
External links
- Oxalic acid MS Spectrum
- International Chemical Safety Card 0529
- NIOSH Guide to Chemical Hazards (CDC)
- "Oxalic acid". ChemicalLand21.com.
- Table: Oxalic acid content of selected vegetables (USDA)
- Alternative link: Table: Oxalic Acid Content of Selected Vegetables (USDA)
- About rhubarb poisoning (The Rhubarb Compendium)
- Oxalosis & Hyperoxaluria Foundation (OHF) The Oxalate Content of Food 2008 (PDF)
- Oxalosis & Hyperoxaluria Foundation (OHF) Diet Information
- Calculator: Water and solute activities in aqueous oxalic acid