Plasmodesma
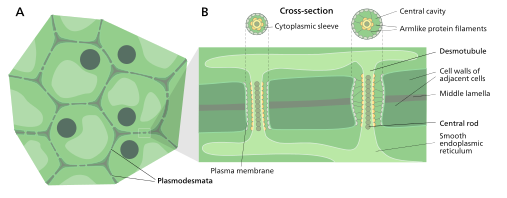


Plasmodesmata (singular: plasmodesma) are microscopic channels which traverse the cell walls of plant cells[2][3] and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages,[4] and species that have these structures include members of the Charophyceae, Charales, Coleochaetales and Phaeophyceae (which are all algae), as well as all embryophytes, better known as land plants.[5] Unlike animal cells, every plant cell is surrounded by a polysaccharide cell wall. Neighbouring plant cells are therefore separated by a pair of cell walls and the intervening middle lamella, forming an extracellular domain known as the apoplast. Although cell walls are permeable to small soluble proteins and other solutes, plasmodesmata enable direct, regulated, symplastic intercellular transport of substances between cells. There are two forms of plasmodesmata: primary plasmodesmata, which are formed during cell division, and secondary plasmodesmata, which can form between mature cells.[6]
Similar structures, called gap junctions[7] and membrane nanotubes, interconnect animal cells[8] and stromules form between plastids in plant cells.[9]
Formation
Plasmodesmata are formed when portions of the endoplasmic reticulum are trapped across the middle lamella as new cell wall is laid down between two newly divided plant cells and these eventually become the cytoplasmic connections between cells (primary plasmodesmata). Here the wall is not thickened further, and depressions or thin areas known as pits are formed in the walls. Pits normally pair up between adjacent cells. Alternatively, plasmodesmata can be inserted into existing cell walls between non-dividing cells (secondary plasmodesmata)[10]
Structure
Plasmodesmatal plasma membrane
A typical plant cell may have between 103 and 105 plasmodesmata connecting it with adjacent cells[11] equating to between 1 and 10 per µm2.[12] Plasmodesmata are approximately 50-60 nm in diameter at the midpoint and are constructed of three main layers, the plasma membrane, the cytoplasmic sleeve, and the desmotubule.[11] They can transverse cell walls that are up to 90 nm thick.[12]
The plasma membrane portion of the plasmodesma is a continuous extension of the cell membrane or plasmalemma [13] It is similar in structure to the cellular phospholipid bilayers.
Cytoplasmic sleeve
The cytoplasmic sleeve is a fluid-filled space enclosed by the plasmalemma and a continuous extension of the cytosol. Trafficking of molecules and ions through plasmodesmata occurs through this passage. Smaller molecules (e.g. sugars and amino acids) and ions can easily pass through plasmodesmata by diffusion without the need for additional chemical energy. Larger molecules, including proteins (for example Green fluorescent protein) and RNA, can also pass through the cytoplasmic sleeve diffusively.[14] Plasmodesmal transport of some larger molecules is facilitated by unknown mechanisms. One main mechanism of regulation of plasmodesmal transport is the accumulation of the polysaccharide callose accumulates around the neck region of plasmodesmata to form a collar, reducing their diameter and thereby controlling permeability to substances in the cytoplasm.[13]
Desmotubule
The desmotubule is a tube of appressed endoplasmic reticulum that runs between two adjacent cells [15] Some molecules are known to be transported through this channel,[16] but it is not thought to be the main route for plasmodesmatal transport.
Around the desmotubule and the plasma membrane areas of an electron dense material have been seen, often joined together by spoke-like structures that seem to split the plasmodesma into smaller channels [15] These structures may be composed of myosin[17][18][19] and actin,[18][20] which are part of the cell's cytoskeleton. If this is the case these proteins could be used in the selective transport of large molecules between the two cells.
Transport

Plasmodesmata have been shown to transport proteins (including transcription factors), short interfering RNA, messenger RNA and viral genomes from cell to cell. One example of a viral movement proteins is the tobacco mosaic virus MP-30. MP-30 is thought to bind to the virus's own genome and shuttle it from infected cells to uninfected cells through plasmodesmata.[14] Flowering Locus T protein moves from leaves to the shoot apical meristem through plasmodesmata to initiate flowering.[21]
Plasmodesmata are also used by cells in phloem, and symplastic transport is used to regulate the sieve-tube cells by the companion cells.[22]
The size of molecules that can pass through plasmodesmata is determined by the size exclusion limit. This limit is highly variable and can is subject to active modification.[6] MP-30 is able to increase the size exclusion limit from 700 Daltons to 9400 Daltons thereby aiding its movement through a plant.[23]
Several models for possible active transport through plasmodesmata exist. It has been suggested that such transport is mediated by interactions with proteins localized on the desmotubule, and/or by chaperones partially unfolding proteins, allowing them to fit through the narrow passage. A similar mechanism may be involved in transporting viral nucleic acids through the plasmodesmata.[24]
See also
References
- ^ Maule, Andrew (December 2008). "Plasmodesmata: structure, function and biogenesis". Current Opinion in Plant Biology. 11 (6): 680–686. doi:10.1016/j.pbi.2008.08.002. PMID 18824402.
{{cite journal}}:|access-date=requires|url=(help) - ^ Oparka, K. J. (2005) Plasmodesmata. Blackwell Pub Professional. ISBN 1-4051-2554-3 ISBN 978-1-4051-2554-3
- ^ Plasmodesmata (www.dictionary.com)
- ^ http://public.wsu.edu/~lange-m/Documnets/Teaching2011/Popper2011.pdf
- ^ Graham, LE; Cook, ME; Busse, JS (2000), Proceedings of the National Academy of Sciences 97, 4535-4540.
- ^ a b http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1692983 The shoot apical meristem: the dynamics of a stable structure. Jan Traas and Teva Vernoux : Philos Trans R Soc Lond B Biol Sci. 2002 June 29; 357(1422): 737–747. (page 744)
- ^ Bruce Alberts (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1.
- ^ Gallagher KL, Benfey PN (January 2005). "Not just another hole in the wall: understanding intercellular protein trafficking". Genes Dev. 19 (2): 189–95. doi:10.1101/gad.1271005. PMID 15655108.
- ^ Gray JC, Sullivan JA, Hibberd JM, Hansen MR (2001). "Stromules: mobile protrusions and interconnections between plastids". Plant Biology. 3: 223–33. doi:10.1055/s-2001-15204.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lucas W., Ding, B. and Van der Schoot, C. (1993) Tansley Review No.58 "Plasmodesmata and the supracellular Nature of Plants" New Phytologist, Vol. 125, No. 3, pp. 435-476, Stable URL: http://www.jstor.org/stable/2558257
- ^ a b AW Robards (1975) Plasmodesmata. Annual Review of Plant Physiology 26, 13-29
- ^ a b "22". Molecular Cell Biology (4 ed.). 2000. p. 998. ISBN 0-7167-3706-X.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b AW Robards (1976) Plasmodesmata in higher plants. In: Intercellular communications in plants: studies on plasmodesmata. Edited by BES Gunning and AW Robards Springer-Verlag Berlin pps 15-57.
- ^ a b http://www.ingentaconnect.com/content/bsc/pce/2003/00000026/00000001/art00007 Plasmodesmata and the control of symplastic transport A. G. ROBERTS & K. J. OPARKA
- ^ a b RL Overall, J Wolfe, BES Gunning (1982) Intercellular communication in Azolla roots: I. Ultrastructure of plasmodesmata. Protoplasma 111: 134-150
- ^ LC Cantrill, RL Overall and PB Goodwin (1999) Cell-to-cell communication via plant endomembranes. Cell Biology International 23: 653–661
- ^ JE Radford and RG White (1998) Localization of a myosin‐like protein to plasmodesmata. Plant Journal 14: 743-750
- ^ a b LM Blackman and RL Overall (1998) Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant Journal 14: 733-741
- ^ S Reichelt, AE Knight, TP Hodge, F Baluska, J Samaj, D Volkmann and J Kendrick-Jones (1999) Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant Journal 19: 555–569
- ^ RG White, K Badelt, RL Overall and M Vesk (1994) Actin associated with plasmodesmata. Protoplasma 180: 169-184
- ^ Corbesier, L., Vincent, C., Jang, S., Fornara, F., Fan, Q.; et al. (2007). "FT protein movement contributes to long distance signalling in floral induction of Arabidopsis". Science. 316 (5827): 1030–1033. doi:10.1126/science.1141752. PMID 17446353.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Phloem
- ^ http://www.sciencemag.org/cgi/content/abstract/246/4928/377 Science 20 October 1989: Vol. 246. no. 4928, pp. 377 - 379 Movement Protein of Tobacco Mosaic Virus Modifies Plasmodesmatal Size Exclusion Limit SHMUEL WOLF, WILLIAM J. LUCAS, CARL M. DEOM,and ROGER N. BEACHY
- ^ http://jpkc.zju.edu.cn/k/437/content/05.pdf Plant Physiology lectures, chapter 5
