Esophageal cancer
| Esophageal cancer | |
|---|---|
| Specialty | Oncology, general surgery, gastroenterology |
Esophageal cancer (or oesophageal cancer) is cancer arising from the foodpipe known as the esophagus that runs between the throat and the stomach.[1] Symptoms often include difficulty in swallowing and weight loss. Other symptoms may include pain when swallowing, a hoarse voice, enlarged lymph nodes (glands) around the collarbone, a dry cough, and possibly coughing up or vomiting blood.[2]
The two main sub-types of the disease are esophageal squamous-cell carcinoma (often abbreviated to ESCC),[3] which is more common in the developing world, and esophageal adenocarcinoma (or EAC), which is more common in the developed world.[1] A number of less common types also occur.[1] Squamous-cell carcinoma arises from the skin cells that line the esophagus.[4] Adenocarcinoma arises from glandular cells present in the lower third of the esophagus, often where they have already transformed to intestinal cell type (a condition known as Barrett's esophagus).[1][5] The most common causes of the squamous-cell type are: tobacco, alcohol, very hot drinks, and a poor diet.[6] The most common causes of the adenocarcinoma type are smoking tobacco, obesity, and acid reflux.[6]
The disease is diagnosed by biopsy done by an endoscope (a fiberoptic camera).[7] Prevention includes stopping smoking and a healthy diet.[1][2] Treatment is based on the cancer's stage and location, together with the person's general condition and individual preferences. Small localized squamous-cell cancers may be treated with surgery alone with the hope of a cure. In most other cases, chemotherapy with or without radiation therapy is used along with surgery.[7] Larger tumors may have their growth slowed with chemotherapy and radiation therapy.[1] In the presence of extensive disease or if the affected person is not fit enough to undergo surgery, palliative care is often recommended.[7] Outcomes are related to the extent of the disease and other medical conditions, but generally tend to be fairly poor, as diagnosis is often late.[1][8] Five-year survival rates are around 13% to 18%.[2][9]
As of 2012, esophageal cancer is the eighth-most common cancer globally with 456,000 new cases during the year.[1] It caused about 400,000 deaths that year, up from 345,000 in 1990.[1][10] Rates vary widely between countries, with about half of all cases occurring in China. It is around three times more common in men than in women.[1]
Signs and symptoms
Prominent symptoms usually do not appear until the cancer has infiltrated over 60% of the circumference of the esophageal tube, by which time the tumor is already in an advanced stage.[11] Onset of symptoms is usually caused by narrowing of the tube due to the physical presence of the tumor.[12]
The first and the most common symptom is usually difficulty in swallowing, which is often experienced first with solid foods and later with softer foods and liquids.[2] Pain when swallowing is less usual at first.[2] Weight loss is often an initial symptom in cases of squamous-cell carcinoma, though not usually in cases of adenocarcinoma.[13] Eventual weight loss due to reduced appetite and undernutrition is common.[14] Pain behind the breastbone or in the region around the stomach often feels like heartburn. The pain can frequently be severe, worsening when food of any sort is swallowed. Another sign may be an unusually husky, raspy, or hoarse-sounding cough, a result of the tumor affecting the recurrent laryngeal nerve.
The presence of the tumor may disrupt the normal contractions of the esophagus when swallowing. This can lead to nausea and vomiting, regurgitation of food and coughing.[11] There is also an increased risk of aspiration pneumonia[11] due to food entering the airways through the abnormal connections (fistulas) that may develop between the esophagus and the trachea (windpipe).[8] Early signs of this serious complication may be coughing on drinking or eating.[15] The tumor surface may be fragile and bleed, causing vomiting of blood. Compression of local structures occurs in advanced disease, leading to such problems as upper airway obstruction and superior vena cava syndrome. Hypercalcemia (excess calcium in the blood) may occur.[11]
If the cancer has spread elsewhere, symptoms related to metastatic disease may appear. Common sites of spread include nearby lymph nodes, the liver, lungs and bone.[11] Liver metastasis can cause jaundice and abdominal swelling (ascites). Lung metastasis can cause, among other symptoms, impaired breathing due to excess fluid around the lungs (pleural effusion), and dyspnea (the feelings often associated with impaired breathing).
Causes and protective factors
The two main types (i.e. squamous-cell carcinoma and adenocarcinoma) have distinct sets of risk factors.[13] Squamous-cell carcinoma is linked to lifestyle factors such as smoking and alcohol.[16] Adenocarcinoma has been linked to effects of long-term acid reflux.[16] Tobacco is a risk factor for both types.[13] Both types are more common in men and in the over-60s.[17]
Squamous-cell carcinoma
The two major risk factors for esophageal squamous-cell carcinoma are tobacco (smoking or chewing) and alcohol.[1] The combination of tobacco and alcohol has a strong synergistic effect.[18] Some data suggest that about half of all cases are due to tobacco and about one-third to alcohol, while over three-quarters of the cases in men are due to the combination of smoking and heavy drinking.[1] The risks associated with alcohol appear to be linked to its aldehyde metabolite and to mutations in certain related enzymes.[13] Such metabolic variants are relatively common in Asia.[1]
High levels of dietary exposure to nitrosamines (chemical compounds found both in tobacco smoke and certain foodstuffs) appear to be a relevant risk factor.[13] Unfavorable dietary patterns seem to involve exposure to nitrosamines through processed and barbecued meats, pickled vegetables, etc., and a low intake of fresh foods.[1] Other associated factors include nutritional deficiencies, low socioeconomic status, and poor oral hygiene.[13] Chewing betel nut (areca) is an important risk factor in Asia.[19]
Adenocarcinoma
Male predominance is particularly strong in this type of esophageal cancer, which occurs about 7 to 10 times more frequently in men.[20] This imbalance may be related to the characteristics and interactions of other known risk factors, including acid reflux and obesity.[20]
The long-term erosive effects of acid reflux (an extremely common condition, also known as gastroesophageal reflux disease or GERD) have been strongly linked to this type of cancer.[21] Longstanding GERD can induce a change of cell type in the lower portion of the esophagus in response to erosion of its squamous lining.[21] This phenomenon, known as Barrett's esophagus, seems to appear about 20 years later in women than in men, maybe due to hormonal factors.[21] Having symptomatic GERD or bile reflux makes Barrett's esophagus more likely, which in turn raises the risk of further changes that can ultimately lead to adenocarcinoma.[13] The risk of developing adenocarcinoma in the presence of Barrett's esophagus is unclear, and may in the past have been overestimated.[1]
Obesity and overweight both appear to be associated with increased risk.[22] The association with obesity seems to be the strongest of any type of obesity-related cancer, though the reasons for this remain unclear.[23] Abdominal obesity seems to be of particular relevance, given the closeness of its association with this type of cancer, as well as with both GERD and Barrett's esophagus.[23] This type of obesity is characteristic of men.[23] Physiologically, it stimulates GERD and also has other chronic inflammatory effects.[21]
EAC has one significant protective factor reducing risk for both sexes.
This article needs additional citations for verification. (October 2014) |
Although the Helicobacter pylori bacterium, which has infected over half of the world's population, is a cause of GERD and a risk factor for gastric cancer, it seems to be associated with a reduced risk of esophageal adenocarcinoma of as much as 50%.[24][25] The biological explanation for a protective effect is somewhat unclear.[25] One explanation is that some strains of H. pylori reduce stomach acid, thereby reducing damage by GERD.[26] The decreasing rates of H. pylori infection in Western populations in recent decades have been suggested as a factor in the great increase in oesophageal adenocarcinoma over the same period. The decrease is caused by better hygiene, for example through increased refrigeration of food and less crowded households, and has also been associated with an increase in stomach cancer.[24]
Female hormones may also have a protective effect, as EAC is not only much less common in women but develops later in life, by an average of 20 years. Although studies of many reproductive factors have not produced a clear picture, risk seems to decline for the mother in line with prolonged periods of breastfeeding.[24]
Tobacco smoking increases risk, but the effect in esophageal adenocarcinoma is slight compared to that in squamous cell carcinoma, and alcohol has not been demonstrated to be a cause.[24]
Related conditions
- Head and neck cancer is associated with second primary tumors in the region, including esophageal squamous-cell carcinomas, due to field cancerization (i.e. a regional reaction to long-term carcinogenic exposure).[27][28]
- History of radiation therapy for other conditions in the chest is a risk factor for esophageal adenocarcinoma.[13]
- Corrosive injury to the esophagus by accidentally or intentionally swallowing caustic substances is a risk factor for squamous cell carcinoma.[1]
- Tylosis with esophageal cancer is a rare familial disease that has been linked to a mutation in the RHBDF2 gene: it involves thickening of the skin of the palms and soles and a high lifetime risk of squamous cell carcinoma.[1][29]
- Achalasia (i.e. lack of the involuntary reflex in the esophagus after swallowing) appears to be a risk factor for both main types of esophageal cancer, at least in men, due to stagnation of trapped food and drink.[30]
- Plummer–Vinson syndrome (a rare disease that involves esophageal webs) is also a risk factor.[1]
- There is some evidence suggesting a possible causal association between human papillomavirus (HPV) and esophageal squamous-cell carcinoma.[31] The relationship is unclear.[32] Possible relevance of HPV could be greater in places that have a particularly high incidence of this form of the disease,[33] as in some Asian countries, including China.[34]
- There is limited evidence to support an association between celiac disease and esophageal cancer.[30]
Diagnosis
Clinical evaluation
Although an occlusive tumor may be suspected on a barium swallow or barium meal, the diagnosis is best made with esophagogastroduodenoscopy (endoscopy); this involves the passing of a flexible tube with a light and camera down the esophagus and examining the wall. Biopsies taken of suspicious lesions are then examined histologically for signs of malignancy.
Additional testing is usually performed to estimate the tumor stage, for which the TNM staging system is used. Computed tomography (CT) of the chest, abdomen and pelvis can evaluate whether the cancer has spread to adjacent tissues or distant organs (especially liver and lymph nodes). The sensitivity of a CT scan is limited by its ability to detect masses (e.g. enlarged lymph nodes or involved organs) generally larger than 1 cm. Positron emission tomography is also used to estimate the extent of the disease and is regarded as more precise than CT alone. Esophageal endoscopic ultrasound can provide staging information regarding the level of tumor invasion, and possible spread to regional lymph nodes.
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 in long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur nearer the stomach and squamous cell carcinomas nearer the throat, but either may arise anywhere in the esophagus.
Classification
Esophageal cancers are typically carcinomas which arise from the epithelium, or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: squamous-cell carcinomas, which are similar to head and neck cancer in their appearance and association with tobacco and alcohol consumption, and adenocarcinomas, which are often associated with a history of GERD and Barrett's esophagus. A general rule of thumb is that a cancer in the upper two-thirds is likely to be a squamous-cell carcinoma and one in the lower one-third an adenocarcinoma.
Rare histologic types of esophageal cancer are different variants of the squamous-cell carcinoma, and nonepithelial tumors, such as leiomyosarcoma, malignant melanoma, rhabdomyosarcoma, lymphoma, and others.[35][36]
-
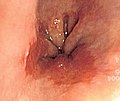
Endoscopic image of an esophageal adenocarcinoma
-
Endoscopic image of Barrett esophagus – a frequent precursor of esophageal adenocarcinoma
-
Endoscopy and radial endoscopic ultrasound images of a submucosal tumor in the central portion of the esophagus
-
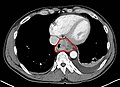
Contrast CT scan showing an esophageal tumor (axial view)
-
Contrast CT scan showing an esophageal tumor (coronal view)
-
Micrograph showing histopathological appearance of an esophageal adenocarcinoma (dark blue – upper-left of image) and normal squamous epithelium (upper-right of image) at H&E staining
Prevention
Prevention includes stopping smoking or chewing tobacco.[1] Overcoming addiction to areca chewing in Asia is another promising strategy for the prevention of esophageal squamous-cell carcinoma.[19]
According to the National Cancer Institute, "diets high in cruciferous (cabbage, broccoli/broccolini, cauliflower, Brussels sprouts) and green and yellow vegetables and fruits are associated with a decreased risk of esophageal cancer."[37] Dietary fiber is thought to be protective, especially against esophageal adenocarcinoma.[38]
Screening
People with Barrett esophagus (a change in the cells lining the lower esophagus) are at much higher risk,[39] and may receive regular endoscopic screening for the early signs of cancer.[40] Because the benefit of screening for adenocarcinoma in people without symptoms is unclear,[1] it is not recommended in the United States.[2] Some areas of the world with high rates of squamous-carcinoma have screening programs.[1]
Management


The treatment is determined by the cellular type of cancer (adenocarcinoma or squamous-cell carcinoma vs other types), the stage of the disease, the general condition of the patient, and other diseases present. Treatment should be managed by an multidisciplinary team covering the various specialities involved.[41][42] Adequate nutrition needs to be assured, and adequate dental care is vital.
A number of types and combinations of surgery, chemotherapy and radiotherapy, as well as other therapies, have been used over recent decades, but there has been no transformative improvement in outlook for patients equivalent to that seen in some other cancers.
Surgery
Early-stage adenocarcinomas will be treated by surgery where this is possible, although esophagectomy, the removal of all or part of the esophagus, is a difficult operation with a relatively high risk of mortality or post-operative difficulties; the benefits of surgery are less clear for early-stage squamous cancers. There are a number of surgical options, and the best choices for particular situations remain the subject of research and discussion.[41][43][44] As well as the characteristics and location of the tumor, other factors include the condition of the patient, and the type of operation to which the surgical team are most used. Surgical outcomes are likely to be better in large centers where the procedures are frequently performed.[43] If the cancer has spread to other parts of the body, esophagectomy will now not normally be performed.[43][45]
Endoscopic mucosal resection (EMR) is the removal of small tumors that only involve the mucosa or lining of the esophagus (stage T1a).[46]
Esophagectomy is the removal of a segment of the esophagus; as this shortens the length of the remaining esophagus, some other segment of the digestive tract is pulled up through the chest cavity and interposed. This is usually the stomach or part of the large intestine (colon) or the jejunum. The joining up of the stomach to a shortened esophagus is called an esophagogastric anastomosis.[43]
There are a number of different methods of esophagectomy; which is used will depend on the nature and location of the tumor, and the preference of the surgeon. Clear evidence from clinical trials for which approaches give the best outcomes in different circumstances is lacking.[43] The first distinction, based on how the surgeon enters the body, is between a transhiatial procedure as opposed to a transthoracic. The more recent transhiatial approach avoids the need to open the chest, instead the surgeon enters the body through an incision in the lower abdomen and another in the neck. The esophagus is freed from the surrounding tissues and cut away as necessary. The stomach is then pushed through the esophageal hiatus, the hole in the diaphragm for the esophagus, and joined to the remaining upper part of the esophagus at the neck.[43]
The traditional transthoracic approach enters the body through the chest, and has a number of variations: the thoracoabdominal approach opens the abdominal and thoracic cavities together, the two-stage Ivor Lewis (also called Lewis–Tanner) approach involves an initial laparotomy and construction of a gastric tube, followed by a right thoracotomy to excise the tumor and create an esophagogastric anastomosis, the three-stage McKeown approach adds a third incision in the neck to complete the cervical anastomosis. Recent approaches by some surgeons use what is called extended esophagectomy, where more surrounding tissue including lymph nodes is removed en bloc.[43]
If the person cannot swallow at all, an esophageal stent may be inserted to keep the esophagus open; stents may also assist in occluding fistulas. A nasogastric tube may be necessary to continue feeding while treatment for the tumor is given, and some patients require a gastrostomy (feeding hole in the skin that gives direct access to the stomach). The latter two are especially important if the patient tends to aspirate food or saliva into the airways, predisposing for aspiration pneumonia.
Chemotherapy and radiotherapy
Chemotherapy depends on the tumor type, but tends to be cisplatin-based (or carboplatin or oxaliplatin) every three weeks with fluorouracil (5-FU) either continuously or every three weeks. In more recent studies, addition of epirubicin was better than other comparable regimens in advanced nonresectable cancer.[47] Chemotherapy may be given after surgery (adjuvant, i.e. to reduce risk of recurrence), before surgery (neoadjuvant) or if surgery is not possible; in this case, cisplatin and 5-FU are used. Ongoing trials compare various combinations of chemotherapy; the phase II/III REAL-2 trial – for example – compares four regimens containing epirubicin and either cisplatin or oxaliplatin, and either continuously infused fluorouracil or capecitabine.
Radiotherapy is given before, during, or after chemotherapy or surgery, and sometimes on its own to control symptoms. In patients with localised disease but contraindications to surgery, "radical radiotherapy" may be used with curative intent.
Other approaches
Forms of endoscopic therapy have been used for stage 0 and I disease: endoscopic mucosal resection (EMR) [46] and mucosal ablation using radiofrequency ablation, photodynamic therapy, Nd-YAG laser, or argon plasma coagulation.
Laser therapy is the use of high-intensity light to destroy tumor cells; it affects only the treated area. This is typically done if the cancer cannot be removed by surgery. The relief of a blockage can help with pain and difficulty swallowing. Photodynamic therapy, a type of laser therapy, involves the use of drugs that are absorbed by cancer cells; when exposed to a special light, the drugs become active and destroy the cancer cells.
Radiofrequency ablation is a new treatment modality for the treatment of Barrett's esophagus and dysplasia, and has been the subject of numerous published clinical trials. The findings demonstrate radiofrequency ablation has an efficacy of 80–90% or greater with respect to complete clearance of Barrett's esophagus and dysplasia with durability up to five years and a favorable safety profile.[48][49][50][51] Recent clinical trials have shown that endoscopic resection of esophageal mucosal irregularities and nodules which contain dysplasia or carcinoma combined with subsequent radiofrequency ablation of the remaining flat Barrett's esophagus and dysplasia can effectively and safely eradicate the disease.[52] Further, a recent multicenter randomized control trial found that in patients with Barrett's esophagus containing nodules or mucosal irregularities which contained high grade dysplasia or cancer, subsequent radiofrequency ablation resulted not only in eradication of Barrett's esophagus and dysplasia, but also had significantly less esophageal stricture versus patients who had circumferential endoscopic mucosal resection for their disease.[51]
-
Internal radiotherapy for esophageal cancer
-
Self-expandable metallic stents are sometimes used for palliative care
Follow-up
Patients are followed up frequently after a treatment regimen has been completed. Frequently, other treatments are necessary to improve symptoms and maximize nutrition.
Prognosis
In general, the prognosis of esophageal cancer is quite poor, because most patients present with advanced disease. By the time the first symptoms (such as difficulty swallowing) appear, the cancer has already well progressed. The overall five-year survival rate (5YSR) in the United States is around 15%, with most people dying within the first year of diagnosis.[53] The latest survival data for England and Wales (patients diagnosed during 2007) show that only one in ten people will survive esophageal cancer for at least ten years.[54]
Individualized prognosis depends largely on stage. Those with cancer restricted entirely to the esophageal mucosa have about an 80% 5YSR, but submucosal involvement brings this down to less than 50%. Extension into the muscularis propria (muscle layer of the esophagus) suggests a 20% 5YSR, and extension to the structures adjacent to the esophagus predict a 7% 5YSR. Patients with distant metastases (who are not candidates for curative surgery) have a less than 3% 5YSR. [citation needed]
-
T1, T2, and T3 stages of esophageal cancer
-
Stage T4 esophageal cancer
-
Esophageal cancer in the lymph nodes
Epidemiology

Esophageal cancer is the eighth most frequently diagnosed cancer worldwide,[1] and because of its poor prognosis it is the sixth most common cause of cancer-related death.[39] It caused about 400,000 deaths in 2012, accounting for about 5% of all cancer deaths (about 456,000 new cases were diagnosed, representing about 3% of all cancers).[1]
ESCC comprises 60–70% of all cases of esophageal cancer worldwide, while EAC accounts for a further 20–30% (melanomas, leiomyosarcomas, carcinoids and lymphomas are less common types).[56] The incidence of the two main types of esophageal cancer varies greatly between different geographical areas.[57]
The worldwide incidence rate of ESCC in 2012 was 5.2 new cases per 100,000 person-years, with a male predominance (7.7 per 100,000 in men vs. 2.8 in women).[58] It was the common type in 90% of the countries studied.[58] ESCC is particularly frequent in the so-called "Asian esophageal cancer belt", an area that passes through northern China, southern Russia, north-eastern Iran, northern Afghanistan and eastern Turkey.[59] In 2012, about 80% of ESCC cases worldwide occurred in central and south-eastern Asia, and over half (53%) of all cases were in China.[58] The countries with the highest estimated national incidence rates were (in Asia) Mongolia and Turkmenistan and (in Africa) Malawi, Kenya and Uganda.[58] The problem of esophageal cancer has long been recognized in the eastern and southern parts of Sub-Saharan Africa, where ESCC appears to predominate.[60]
In Western countries, EAC has become the dominant form of the disease, following an increase in incidence over recent decades (in contrast to the incidence of ESCC, which has remained largely stable).[7][24] In 2012, the global incidence rate for EAC was 0.7 per 100,000 with a strong male predominance (1.1 per 100,000 in men vs. 0.3 in women) Areas with particularly high incidence rates include northern and western Europe, north America and Oceania. The countries with highest recorded rates were the UK, Netherlands, Eire, Iceland and New Zealand.[58]
USA
In the United States, esophageal cancer is the seventh-leading cause of cancer death among males (making up 4% of the total).[61] The National Cancer Institute estimated there were about 18,000 new cases and more than 15,000 deaths from esophageal cancer in 2013 (the American Cancer Society estimated that during 2014, about 18,170 new esophageal cancer cases will be diagnosed, resulting in 15,450 deaths).[57][61] The squamous-cell carcinoma type is more common among African American males with a history of heavy smoking or alcohol use. Until the 1970s, squamous-cell carcinoma accounted for the vast majority of esophageal cancers in the United States. In recent decades, incidence of adenocarcinoma of the esophagus (which is associated with Barrett's esophagus) steadily rose in the United States to the point that it has now surpassed squamous-cell carcinoma. In contrast to squamous-cell carcinoma, esophageal adenocarcinoma is more common in Caucasian men (over the age of 60) than it is in African Americans. Multiple reports indicate esophageal adenocarcinoma incidence has increased during the past 20 years, especially in non-Hispanic white men. Esophageal adenocarcinoma age-adjusted incidence increased in New Mexico from 1973 to 1997. This increase was found in non-Hispanic whites and Hispanics and became predominant in non-Hispanic whites.[62] Esophageal cancer incidence and mortality rates for African Americans continue to be higher than the rate for Causasians. However, incidence and mortality of esophageal cancer has significantly decreased among African Americans since the early 1980s, whereas with Caucasians, it has slightly increased.[63] Between 1975 and 2004, incidence of the adenocarcinoma type increased among white American males by over 460% and among white American females by 335%.[57]
UK
The incidence of esophageal adenocarcinoma has risen considerably in the UK in recent decades.[13] Overall, esophageal cancer is the thirteenth most common cancer in the UK (around 8,300 people were diagnosed with the disease in 2011), and it is the sixth most common cause of cancer death (around 7,700 people died in 2012).[64]
Research directions
This section needs expansion. You can help by adding to it. (August 2014) |
The risk of esophageal squamous-cell carcinoma may be reduced in people using aspirin or related NSAIDs,[65] but in the absence of randomized controlled trials the current evidence is inconclusive.[1][24]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Montgomery, EA; et al. (2014). "Oesophageal Cancer". In Stewart, BW; Wild, CP (ed.). World Cancer Report 2014. World Health Organization. pp. 528–543. ISBN 9283204298.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: editors list (link) - ^ a b c d e f Ferri, FF, ed. (2012). "Esophageal Tumors". Ferri's clinical advisor 2013. Philadelphia, PA: Mosby (Elsevier). pp. 389–391. ISBN 9780323083737.
- ^ Even by those using the British English spelling "oesophagus"
- ^ Kelsen, David (2007). Gastrointestinal oncology: principles and practices (2nd ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 4. ISBN 9780781776172.
- ^ Whittemore, edited by David Schottenfeld, Joseph F. Fraumeni, Jr.; associate editors, Graham A. Colditz, Jonathan M. Samet, Alice S. (2006). Cancer epidemiology and prevention (3rd ed.). Oxford: Oxford University Press. p. 697. ISBN 9780199747979.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b Zhang, HZ; Jin, GF; Shen, HB (Jun 2012). "Epidemiologic differences in esophageal cancer between Asian and Western populations". Chinese journal of cancer. 31 (6): 281–6. doi:10.5732/cjc.011.10390. PMC 3777490. PMID 22507220.
- ^ a b c d Stahl, M; Mariette, C; Haustermans, K; Cervantes, A; Arnold, D; ESMO Guidelines Working, Group (Oct 2013). "Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 24 Suppl 6: vi51-6. doi:10.1093/annonc/mdt342. PMID 24078662.
- ^ a b Enzinger PC, Mayer RJ (2003). "Esophageal cancer" (PDF). N. Engl. J. Med. 349 (23): 2241–52. doi:10.1056/NEJMra035010. PMID 14657432.
- ^ "SEER Stat Fact Sheets: Esophageal Cancer". National Cancer Institute. Retrieved 18 June 2014.
- ^ Lozano, R; Naghavi, M; Foreman, K; Lim, S; Shibuya, K; Aboyans, V; Abraham, J; Adair, T; Aggarwal, R (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c d e Mayer, Robert J (2008). "Gastrointestinal Tract Cancer". In Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. (ed.). Harrison's principles of internal medicine. Vol. 1 (18th ed.). New York: McGraw-Hill Medical Publishing Division. pp. 764–5. ISBN 978-0071748896.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Cheifetz, Adam S; Brown, Alphonso; Curry, Michael; Moss, Alan C (2011). Oxford American Handbook of Gastroenterology and Hepatology. Oxford University Press. p. 106. ISBN 978-0-19-983012-1.
- ^ a b c d e f g h i Pennathur A, Gibson MK, Jobe BA, Luketich JD (February 2013). "Oesophageal carcinoma". Lancet. 381 (9864): 400–12. doi:10.1016/S0140-6736(12)60643-6. PMID 23374478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yamada, Tadataka (2011). Textbook of Gastroenterology. John Wiley & Sons. pp. 1590–1. ISBN 978-1-4443-5941-1.
- ^ Gerdes, Hans; Ferguson, Mark K (2002). "Palliation of Esophageal Cancer". In Posner, Mitchell C; Vokes, Everett E; Weichselbaum, Ralph R (ed.). Cancer of the Upper Gastrointestinal Tract. PMPH-USA. p. 184. ISBN 978-1-55009-101-4.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b Lao-Sirieix, P; Caldas, C; Fitzgerald, RC (June 2010). "Genetic predisposition to gastro-oesophageal cancer". Current Opinion in Genetics & Development. 20 (3): 210–7. doi:10.1016/j.gde.2010.03.002. PMID 20347291.
- ^ Tobias JS, Hochhauser D (2013). Cancer and its management (6th ed.). p. 254. ISBN 1-11871-325-7.
- ^ Prabhu, A; Obi, KO; Rubenstein, JH (June 2014). "The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis". The American Journal of Gastroenterology. 109 (6): 822–7. doi:10.1038/ajg.2014.71. PMID 24751582.
- ^ a b Akhtar, S (February 2013). "Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies". Cancer Causes & Control. 24 (2): 257–65. doi:10.1007/s10552-012-0113-9. PMID 23224324.
- ^ a b Rutegård M, Lagergren P, Nordenstedt H, Lagergren J (July 2011). "Oesophageal adenocarcinoma: the new epidemic in men?". Maturitas. 69 (3): 244–8. doi:10.1016/j.maturitas.2011.04.003. PMID 21602001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d de Jonge, PJ; van Blankenstein, M; Grady, WM; Kuipers, EJ (January 2014). "Barrett's oesophagus: epidemiology, cancer risk and implications for management". Gut. 63 (1): 191–202. doi:10.1136/gutjnl-2013-305490. PMID 24092861.
- ^ Turati F, Tramacere I, La Vecchia C, Negri E (March 2013). "A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma". Annals of Oncology. 24 (3): 609–17. doi:10.1093/annonc/mds244. PMID 22898040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Lagergren J (June 2011). "Influence of obesity on the risk of esophageal disorders". Nature Reviews. Gastroenterology & Hepatology. 8 (6): 340–7. doi:10.1038/nrgastro.2011.73. PMID 21643038.
- ^ a b c d e f Lagergren, J; Lagergren, P (2013). "Recent developments in esophageal adenocarcinoma". CA: a Cancer Journal for Clinicians. 63 (4): 232–48. doi:10.3322/caac.21185. PMID 23818335.
- ^ a b Falk, GW (July 2009). "Risk factors for esophageal cancer development" (PDF). Surgical Oncology Clinics of North America. 18 (3): 469–85. doi:10.1016/j.soc.2009.03.005. PMID 19500737.
- ^ Harris, Randall E (2013). "Epidemiology of Esophageal Cancer". Epidemiology of Chronic Disease: Global Perspectives. Burlington, MA: Jones & Bartlett Publishers. pp. 157–161. ISBN 978-0-7637-8047-0.
- ^ Priante AV, Castilho EC, Kowalski LP (April 2011). "Second primary tumors in patients with head and neck cancer". Current Oncology Reports. 13 (2): 132–7. doi:10.1007/s11912-010-0147-7. PMID 21234721.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Scherübl H, Steinberg J, Schwertner C, Mir-Salim P, Stölzel U, de Villiers EM (June 2008). "'Field cancerization' im oberen Aerodigestivtrakt". HNO (in German). 56 (6): 603–8. doi:10.1007/s00106-007-1616-7. PMID 17928979.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ "Tylosis with esophageal cancer". rarediseases.info.nih.gov. Genetic and Rare Diseases Information Center (GARD) – NIH. 18 January 2013. Retrieved 16 August 2014.
- ^ a b Nyrén, O; Adami, HO (2008). "Esophageal Cancer". In Adami, HO; Hunter DJ; Trichopoulos D (ed.). Textbook of Cancer Epidemiology. Vol. Volume 1. Oxford University Press. p. 224. ISBN 978-0-19-531117-4.
{{cite book}}:|volume=has extra text (help)CS1 maint: multiple names: editors list (link) - ^ Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A, Macintyre CR (2013). "The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis". Plos One. 8 (7): e69238. doi:10.1371/journal.pone.0069238. PMC 3722293. PMID 23894436.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Sitas F, Egger S, Urban MI, Taylor PR, Abnet CC, Boffetta P, O'Connell DL, Whiteman DC, Brennan P, Malekzadeh R, Pawlita M, Dawsey SM, Waterboer T (January 2012). "InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers". Journal of the National Cancer Institute. 104 (2): 147–58. doi:10.1093/jnci/djr499. PMC 3260131. PMID 22228147.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Syrjänen, K (January 2013). "Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis". Scandinavian Journal of Infectious Diseases. 45 (1): 1–18. doi:10.3109/00365548.2012.702281. PMID 22830571.
- ^ Hardefeldt, HA; Cox, MR; Eslick, GD (June 2014). "Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis". Epidemiology and Infection. 142 (6): 1119–37. doi:10.1017/S0950268814000016. PMID 24721187.
- ^ W Shield, Thomas. LoCicero, Joseph. B. Ponn, Ronald. (2005). Less Common Malignant Tumors of the Esophagus. Lippincott Williams & Wilkins. pp. 2325–2340. ISBN 978-0-7817-3889-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Halperin, Edward C. (2008). Perez and Brady's principles and practice of radiation oncology. Lippincott Williams & Wilkins. p. 1137. ISBN 978-0-7817-6369-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ NCI (2002). "Prevention: Dietary Factors, based on Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer". Nutr Cancer. 44 (2): 104–26. doi:10.1207/S15327914NC4402_01. PMID 12734057.
- ^ Coleman HG, Murray LJ, Hicks B, Bhat SK, Kubo A, Corley DA, Cardwell CR, Cantwell MM (July 2013). "Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis". Nutrition Reviews. 71 (7): 474–82. doi:10.1111/nure.12032. PMID 23815145.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Zhang Y (September 2013). "Epidemiology of esophageal cancer". World J. Gastroenterol. 19 (34): 5598–606. doi:10.3748/wjg.v19.i34.5598. PMC 3769895. PMID 24039351.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Dunbar KB, Spechler SJ (May 2014). "Controversies in Barrett Esophagus". Mayo Clin. Proc. 89 (7): 973–984. doi:10.1016/j.mayocp.2014.01.022. PMID 24867396.
- ^ a b Tobias, Jeffrey S.; Hochhauser, Daniel (2010). Cancer and its Management (6th ed.). p. 257. ISBN 1118713257.
- ^ Berry 2014, p. S292
- ^ a b c d e f g "Ch 79, "Treatment"". DeVita, Hellman, and Rosenberg's Cancer: Cancer: Principles & Practice of Oncology (9th ed.). Lippincott Williams & Wilkins. 2011. ISBN 9781451105452. Online edition, with updates to 2014
- ^ Berry, MF (May 2014). "Esophageal cancer: staging system and guidelines for staging and treatment". Journal of thoracic disease. 6 (Suppl 3): S289–97. PMID 24876933.
{{cite journal}}: Invalid|ref=harv(help) - ^ Berry 2014, p. S293
- ^ a b Wang KK, Prasad G, Tian J (September 2010). "Endoscopic mucosal resection and endoscopic submucosal dissection in esophageal and gastric cancers". Curr. Opin. Gastroenterol. 26 (5): 453–8. doi:10.1097/MOG.0b013e32833e4712. PMC 3215503. PMID 20703112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ross P, Nicolson M, Cunningham D; et al. (April 2002). "Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 20 (8): 1996–2004. doi:10.1200/JCO.2002.08.105. PMID 11956258.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Fleischer DE, Overholt BF, Sharma VK; et al. (October 2010). "Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial". Endoscopy. 42 (10): 781–9. doi:10.1055/s-0030-1255779. PMID 20857372.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Shaheen NJ, Sharma P, Overholt BF; et al. (May 2009). "Radiofrequency ablation in Barrett's esophagus with dysplasia". N. Engl. J. Med. 360 (22): 2277–88. doi:10.1056/NEJMoa0808145. PMID 19474425.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Shaheen NJ, Overholt BF, Sampliner RE; et al. (August 2011). "Durability of radiofrequency ablation in Barrett's esophagus with dysplasia". Gastroenterology. 141 (2): 460–8. doi:10.1053/j.gastro.2011.04.061. PMC 3152658. PMID 21679712.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b van Vilsteren FG, Pouw RE, Seewald S; et al. (June 2011). "Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial". Gut. 60 (6): 765–73. doi:10.1136/gut.2010.229310. PMID 21209124.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Pouw RE, Wirths K, Eisendrath P; et al. (January 2010). "Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia". Clin. Gastroenterol. Hepatol. 8 (1): 23–9. doi:10.1016/j.cgh.2009.07.003. PMID 19602454.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Polednak AP (May 2003). "Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas". Int. J. Cancer. 105 (1): 98–100. doi:10.1002/ijc.11029. PMID 12672037.
- ^ Cancer Research UK http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus/survival/.
{{cite web}}: Missing or empty|title=(help) - ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved Nov 11, 2009.
- ^ Conteduca V, Sansonno D, Ingravallo G, Marangi S, Russi S, Lauletta G, Dammacco F (August 2012). "Barrett's esophagus and esophageal cancer: an overview". International Journal of Oncology. 41 (2): 414–24. doi:10.3892/ijo.2012.1481. PMID 22615011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Napier KJ, Scheerer M, Misra S (May 2014). "Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities". World Journal of Gastrointestinal Oncology. 6 (5): 112–20. doi:10.4251/wjgo.v6.i5.112. PMC 4021327. PMID 24834141.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c d e Arnold M, Soerjomataram I, Ferlay J, Forman D (2014). "Global incidence of oesophageal cancer by histological subtype in 2012". Gut. doi:10.1136/gutjnl-2014-308124. PMID 25320104.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Conteduca V, Sansonno D, Ingravallo G, Marangi S, Russi S, Lauletta G, Dammacco F (August 2012). "Barrett's esophagus and esophageal cancer: an overview". International Journal of Oncology. 41 (2): 414–24. doi:10.3892/ijo.2012.1481. PMID 22615011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kachala R (September 2010). "Systematic review: epidemiology of oesophageal cancer in Sub-Saharan Africa". Malawi Medical Journal. 22 (3): 65–70. PMC 3345777. PMID 21977849.
- ^ a b "Cancer Facts and Figures 2014" (PDF). American Cancer Society. Retrieved 28 April 2014.
- ^ Vega KJ, Jamal MM (September 2000). "Changing pattern of esophageal cancer incidence in New Mexico". Am. J. Gastroenterol. 95 (9): 2352–6. doi:10.1111/j.1572-0241.2000.02329.x. PMID 11007241.
- ^ "Incidence and Mortality Rate Trends" (PDF). A Snapshot of Esophageal Cancer. National Cancer Institute. September 2006. Archived from the original (PDF) on 2007-03-16. Retrieved 2007-03-21.
- ^ "Oesophageal cancer statistics". Cancer Research UK. Retrieved 3 October 2014.
- ^ Sun, L; Yu, S (Nov 2011). "Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma". Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 24 (8): 544–9. doi:10.1111/j.1442-2050.2011.01198.x. PMID 21539676.