Mercury nano-trap water filtration
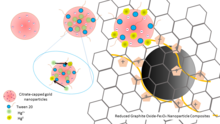
Mercury is considered to be one of the most notorious metal pollutants present in food, water, air and soil, but the process of eliminating it is limited.[1] Heavy metals such as mercury are formed on the earth’s crust and made into solutions with ground water through certain natural processing and pH changes occurring in the soil.[2] There are traditional methods that are used to extract mercury from the natural water sources and industrial waste water, such as chemical precipitation, amalgamation, reverse osmosis, membrane filtration and photochemical methods.[3][4][5] However, these methods are expensive, time consuming, and inefficient, hence the need for a nanofiltration technology that overcomes all of these issues. Nanofiltration technology is very efficient in removal of mercury species due to its characteristics of having high surface area-to-volume and the fact that it’s easily chemically functionalized.[6] Additionally, Brownian motion of nanomaterials allows them to scan large volume of solvent in short times. There are many copolymer nanoparticles (NPs) that can be used as scavengers to eliminate mercury species via redox reactions such as selenium NPs, manganese dioxide nanowhiskers, carbon nanotube−silverNP composites, silver NPs, silver NP-decorated silicaspheres, gold NP-based materials. Among these adsorbents, citrate-capped gold NP-based materials have been used intensively to capture mercury species from nature water.
Instrumentation
High resolution transmission electron microscopy (HRTEM) is used to obtain the HRTEM images (FEI Tecnai G2 F20 S-Twin working at 200 kV) and double beam UV-visible spectrophotometer is used for the extinction, or removal, of the NPs gold spectra.[7] A combination of scanning electron microscope and an energy dispersive X-ray detector (EDX) for obtaining EDX spectra while sub micrometer particle size analyzer and Delsa nano zeta potential measure the potential of gold NPs. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) measures the quantification of mercury species in gold NPs while the linear range of Hg2+ should be between 2.5 and 50 nM. The powder X-ray diffraction is measured using a diffractometer with Cu Kα radiation. A superconducting quantum interference device measures the magnetometry while the Fourier-transformed infrared spectroscopy spectrum is measured by a Nicolet 6700 FT-IR spectrometer.
Removal of Mercury Species
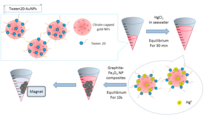
The application of an external magnetic field helps reduce the Fe3O4 NP compositions and can be used to remove the citrate capped gold NPs or Tween 20- Au NPs. The quantity of the Hg2+ is determined by ICP-MS. Elimination efficiency is calculated as:
The equilibrium absorption capacity was determined by:
where qe is the equilibrium absorption capacity, Ce is the concentration of Hg2+, V is the volume of the solution and W is the weight of Tween 20-Au NPs. Other metals in place of mercury can be used to determine the reusability of the nanofiltration method. A simple nanofiltration process is illustrated in Figure 2.

When purifying mercury from sea water, a decrease in the volume to surface area ratio leads to a decline in the efficiency of the elimination.7 Cost efficiency of the nanotrap process is indicated in the fact that the materials can be reused to recycle more water. Tween 20-Au NPs are rapid, efficient and selective in capturing Hg2+ in high salt water concentration when the gold NPs catalyze the citrate ion induced reduction of Hg2+ to Hg0.[8] ICP-MS is used to quantify the Hg2+ concentration to determine the level of elimination efficiency. Figure 3 shows an illustration process of the nano trap filtration process.
The use of nanomaterials in removing the mercury from water is advantageous because of the high surface area to volume ratio and the fact that they are easily chemically functionalized. Nanomaterials capture five times more mercury than the maximum mercury captured predicted through the use of previous mercury filtration systems.[9] Compared to other NP-based methods for Hg2+ removal from high salt matrix, Tween 20-Au NPs is a rapid and selective technique with high efficiency.[10] For purification of drinking water, it is advisable to use nano-tablets with the filters because they are easily accessible and cost efficient.[11][12]
References
- ^ Li B, Zhang Y, Ma D, Shi Z, Ma S. Mercury Nano-Trap for Effective and Efficient Removal of Mercury (II) from Aqueous Solution. Nature Communications 2014 November 20; 5(5537): 1-7.
- ^ Ralston N. Nanomaterials: Nano-selenium captures mercury. Nature Nanotechnology 2008 09;3(9):527-528.
- ^ Tucker P. Purification at the Nano Scale. The Futurist 2011 Jul;45(4):11-12.
- ^ L. W. Chang, “Neurotoxic effects of mercury—A review,” Environmental Research, vol. 14, no. 3, pp. 329–373, 1977.
- ^ Q. Wang, D. Kim, D. D. Dionysiou, G. A. Sorial, and D. Timberlake, “Sources and remediation for mercury contamination in aquatic systems—a literature review,” Environmental Pollution, vol. 131, no. 2, pp. 323–336, 2004.
- ^ Hashim MA, Mukhopadhyay S, Sahu NJ, Sengupta B. Remediation Technologies for Heavy Metal Contaminated Groundwater. Journal of Environmental Management. 2011,June 25; 92(2011): 2355-2388.
- ^ Shih YC, Ke CY, Yu CJ, Lu CY, Tseng WL. Combined tween 20-stabilized gold nanoparticles and reduced graphite oxide-Fe3O4 nanoparticle composites for rapid and efficient removal of mercury species from a complex matrix. ACS Applied Mater Interfaces 2014 October 22; 6(20): 17437-17445.
- ^ F. S. Zhang, J. O. Nriagu, and H. Itoh, “Mercury removal from water using activated carbons derived from organic sewage sludge,” Water Research, vol. 39, no. 2-3, pp. 389–395, 2005.
- ^ Kuriyama M, Haruta K, Dairaku T, Kawamura T, Kikkawa S, Inamoto K, Tsukamoto H, Kondo Y, Torigoe H, Okamoto I, Ono A, Morita EH, Tanaka Y: Hg2+-trapping beads: Hg2+-specific recognition through thymine-Hg(II)-thymine base pairing. Chem Pharm Bull (Tokyo); 2014;62(7):709-12.
- ^ Isaac Ojea-Jiménez, Xicoténcatl López, Jordi Arbiol, and Victor Puntes: Citrate-Coated Gold Nanoparticles As Smart Scavengers for Mercury(II) Removal from Polluted Waters. ACS Nano 2012 6 (3), 2253-2260.
- ^ Heidarpour F, Karim WA, Fakhru'l-razi A, Sobri S, Heydarpour V, Zargar M, et al. Complete removal of pathogenic bacteria from drinking water using nano silver-coated cylindrical polypropylene filters. Clean Technologies and Environmental Policy 2011 06;13(3):499-507.
- ^ Nano-tablet purifies drinking water. Appr Technol 2013 06;40(2):13-14.
This article has not been added to any content categories. Please help out by adding categories to it so that it can be listed with similar articles, in addition to a stub category. (March 2015) |
