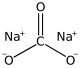
Sodium carbonate
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium carbonate
| |||
| Other names
Soda ash, Washing soda, Soda crystals
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.127 | ||
| E number | E500(i) (acidity regulators, ...) | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Na2CO3 | |||
| Molar mass | 105.9888 g/mol (anhydrous) 286.1416 g/mol (decahydrate) | ||
| Appearance | White solid, hygroscopic | ||
| Odor | Odorless | ||
| Density | 2.54 g/cm3 (25 °C, anhydrous) 1.92 g/cm3 (856 °C) 2.25 g/cm3 (monohydrate)[1] 1.51 g/cm3 (heptahydrate) 1.46 g/cm3 (decahydrate)[2] | ||
| Melting point | 851 °C (1,564 °F; 1,124 K) decomposes (anhydrous) 100 °C (212 °F; 373 K) decomposes (monohydrate) 33.5 °C (92.3 °F; 306.6 K) decomposes (heptahydrate) 34 °C (93 °F; 307 K) (decahydrate)[2][6] | ||
| Decahydrate: 7 g/100 mL (0 °C) 16.4 g/100 mL (15 °C) 34.07 g/100 mL (27.8 °C) Heptahydrate: 48.69 g/100 mL (34.8 °C) Monohydrate: 50.31 g/100 mL (29.9 °C) 48.1 g/100 mL (41.9 °C) 45.62 g/100 mL (60 °C) 43.6 g/100 mL (100 °C)[3] | |||
| Solubility | Soluble in aq. alkalis,[3] glycerol Slightly soluble in aq. alcohol Insoluble in CS2, acetone, alkyl acetates, alcohol, benzonitrile, liquid ammonia[4] | ||
| Solubility in glycerine | 98.3 g/100 g (15.5 °C)[4] | ||
| Solubility in ethanediol | 3.46 g/100 g (20 °C)[5] | ||
| Solubility in dimethylformamide | 0.5 g/kg[5] | ||
| Basicity (pKb) | 3.67 | ||
| −4.1·10−5 cm3/mol[2] | |||
Refractive index (nD)
|
1.485 (anhydrous) 1.420 (monohydrate)[6] 1.405 (decahydrate) | ||
| Viscosity | 3.4 cP (887 °C)[5] | ||
| Structure | |||
| Monoclinic (γ-form, β-form, δ-form, anhydrous)[7] Orthorhombic (monohydrate, heptahydrate)[1][8] | |||
| C2/m, No. 12 (γ-form, anhydrous, 170 K) C2/m, No. 12 (β-form, anhydrous, 628 K) P21/n, No. 14 (δ-form, anhydrous, 110 K)[7] Pca21, No. 29 (monohydrate)[1] Pbca, No. 61 (heptahydrate)[8] | |||
| 2/m (γ-form, β-form, δ-form, anhydrous)[7] mm2 (monohydrate)[1] 2/m 2/m 2/m (heptahydrate)[8] | |||
a = 8.920(7) Å, b = 5.245(5) Å, c = 6.050(5) Å (γ-form, anhydrous, 295 K)[7] α = 90°, β = 101.35(8)°, γ = 90°
| |||
| Octahedral (Na+, anhydrous) | |||
| Thermochemistry | |||
Heat capacity (C)
|
112.3 J/mol·K[2] | ||
Std molar
entropy (S⦵298) |
135 J/mol·K[2] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−1130.7 kJ/mol[2][5] | ||
Gibbs free energy (ΔfG⦵)
|
−1044.4 kJ/mol[2] | ||
| Hazards | |||
| GHS labelling: | |||
 [9] [9]
| |||
| Warning | |||
| H319[9] | |||
| P305+P351+P338[9] | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
4090 mg/kg (rat, oral) [10] | ||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Other anions
|
Sodium bicarbonate | ||
Other cations
|
Lithium carbonate Potassium carbonate Rubidium carbonate Caesium carbonate | ||
Related compounds
|
Sodium sesquicarbonate Sodium percarbonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sodium carbonate (also known as washing soda, soda ash and soda crystals), Na2CO3, is the water-soluble sodium salt of carbonic acid.
It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odourless powder that is hygroscopic (absorbs moisture from the air), has an alkaline taste, and forms a strongly alkaline water solution. Sodium carbonate is well known domestically for its everyday use as a water softener. It can be extracted from the ashes of many plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber (used to create potash), they became known as "soda ash".[12] It is synthetically produced in large quantities from salt (sodium chloride) and limestone by a method known as the Solvay process.
Uses
The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the pre-melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries.
Sodium carbonate is also used as a relatively strong base in various settings. For example, sodium carbonate is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents.
It is a common additive in swimming pools used to neutralize the corrosive effects of chlorine and raise the pH.
In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning.
In taxidermy, sodium carbonate added to boiling water will remove flesh from the skull or bones of trophies to create the "European skull mount" or for educational display in biological and historical studies.
In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.
Domestic use
It is used as a water softener in laundering: it competes with the magnesium and calcium ions in hard water and prevents them from bonding with the detergent being used. Sodium carbonate can be used to remove grease, oil and wine stains.
In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used to ensure proper chemical bonding of the dye with cellulose (plant) fibers, typically before dyeing (for tie dyes), mixed with the dye (for dye painting), or after dyeing (for immersion dyeing).
Sodium carbonate test
The sodium carbonate test (not to be confused with sodium carbonate extract test) is used to distinguish between some common metal ions, which are precipitated as their respective carbonates. The test can distinguish between Cu, Fe and Ca/Zn/Pb. Sodium carbonate solution is added to the salt of the metal. A blue precipitate indicates Cu2+ ion. A dirty green precipitate indicates Fe2+ ion. A yellow-brown precipitate indicates Fe3+ ion. A white precipitate indicates Ca2+, Zn2+ or Pb2+ ion. The compounds formed are, respectively, copper(II) carbonate, iron(II) carbonate, iron(III) oxide, calcium carbonate, zinc carbonate and lead(II) carbonate. This test is used to precipitate the ion present as almost all carbonates are insoluble. While this test is useful for telling these cations apart, it fails if other ions are present, because most metal carbonates are insoluble and will precipitate. In addition, calcium, zinc and lead ions all produce white precipitates with carbonate, making it difficult to distinguish between them. Instead of sodium carbonate, sodium hydroxide may be added, this gives nearly the same colours, except that lead and zinc hydroxides are soluble in excess alkali, and can hence be distinguished from calcium. For the complete sequence of tests used for qualitative cation analysis, see qualitative inorganic analysis.
Other applications
Sodium carbonate is a food additive (E500) used as an acidity regulator, anti-caking agent, raising agent, and stabilizer. It is one of the components of kansui (かん水), a solution of alkaline salts used to give ramen noodles their characteristic flavor and texture. It is also used in the production of snus (Swedish-style snuff) to stabilize the pH of the final product. In Sweden, snus is regulated as a food product because it is put into the mouth, requires pasteurization, and contains only ingredients that are approved as food additives.
Sodium carbonate is also used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
In China, it is used to replace lye-water in the crust of traditional Cantonese moon cakes, and in many other Chinese steamed buns and noodles.
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay.
In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate.
Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid de-linting of fuzzy cottonseed.
Sodium carbonate, in a solution with common salt, may be used for cleaning silver. In a non-reactive container (glass, plastic or ceramic) aluminium foil and the silver object are immersed in the hot salt solution. The elevated pH dissolves the aluminium oxide layer on the foil and enables an electrolytic cell to be established. Hydrogen ions produced by this reaction reduce the sulfide ions on the silver restoring silver metal. The sulfide can be released as small amounts of hydrogen sulfide. Rinsing and gently polishing the silver restores a highly polished condition.[13]
Sodium carbonate is used in some aquarium water pH buffers to maintain a desired pH and carbonate hardness (KH).
Because of its ability to absorb CO2, sodium carbonate is being investigated as a carbon-capturing material for power plants and in other industries that produce greenhouse gases.[14]
Physical properties
The integral enthalpy of solution of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution.[15] The Mohs hardness of sodium carbonate monohydrate is 1.3.[6]
Occurrence
Sodium carbonate crystallizes from water to form three different hydrates:
- sodium carbonate decahydrate (natron)
- sodium carbonate heptahydrate (not known in mineral form)
- sodium carbonate monohydrate (thermonatrite).

Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate. Deposits of the mineral natron have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of mummies and in the early manufacture of glass.
The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from Ol Doinyo Lengai, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past but, due to these minerals' instability at the earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as trona, trisodium hydrogendicarbonate dihydrate, are also known from ultra-alkaline pegmatitic rocks, that occur for example in the Kola Peninsula in Russia.
Production
Mining
Trona, trisodium hydrogendicarbonate dihydrate (Na3HCO3CO3·2H2O), is mined in several areas of the US and provides nearly all the domestic consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near Green River, Wyoming, have made mining more economical than industrial production in North America. There are important reserves of trona in Turkey; two million tons of soda ash have been extracted from the reserves near Ankara. It is also mined from some alkaline lakes such as Lake Magadi in Kenya by dredging. Hot saline springs continuously replenish salt in the lake so that, provided the rate of dredging is no greater than the replenishment rate, the source is fully sustainable.[citation needed]
Barilla and kelp
Several "halophyte" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically glassworts or saltworts) or the seaweed (typically Fucus species) were harvested, dried, and burned. The ashes were then "lixiviated" (washed with water) to form an alkali solution. This solution was boiled dry to create the final product, which was termed "soda ash"; this very old name refers to the archetypal plant source for soda ash, which was the small annual shrub Salsola soda ("barilla plant").
The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("kelp"), to 30 percent for the best barilla produced from saltwort plants in Spain. Plant and seaweed sources for soda ash, and also for the related alkali "potash", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.[16]
Leblanc process
In 1791, the French chemist Nicolas Leblanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal. First, sea salt (sodium chloride) was boiled in sulfuric acid to yield sodium sulfate and hydrogen chloride gas, according to the chemical equation
Next, the sodium sulfate was blended with crushed limestone (calcium carbonate) and coal, and the mixture was burnt, producing calcium sulfide.
The sodium carbonate was extracted from the ashes with water, and then collected by allowing the water to evaporate.
The hydrochloric acid produced by the Leblanc process was a major source of air pollution, and the calcium sulfide byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.[16][17]
Solvay process
In 1861, the Belgian industrial chemist Ernest Solvay developed a method to convert sodium chloride to sodium carbonate using ammonia. The Solvay process centered around a large hollow tower. At the bottom, calcium carbonate (limestone) was heated to release carbon dioxide:
At the top, a concentrated solution of sodium chloride and ammonia entered the tower. As the carbon dioxide bubbled up through it, sodium bicarbonate precipitated:
The sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (calcium hydroxide) left over from carbon dioxide generation:
Because the Solvay process recycles its ammonia, it consumes only brine and limestone, and has calcium chloride as its only waste product. This made it substantially more economical than the Leblanc process, and it soon came to dominate world sodium carbonate production. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
Hou's process
Developed by Chinese chemist Hou Debang in the 1930s. The earlier steam reforming byproduct carbon dioxide was pumped through a saturated solution of sodium chloride and ammonia to produce sodium bicarbonate via the following reactions:
The sodium bicarbonate was collected as a precipitate due to its low solubility and then heated to yield pure sodium carbonate similar to last step of the Solvay process. More sodium chloride is added to the remaining solution of ammonium and sodium chlorides; also more ammonia is pumped at 30-40 °C to this solution. The solution temperature is then lowered to below 10 °C. Solubility of ammonium chloride is higher than that of sodium chloride at 30 °C and lower at 10 °C. Due to this temperature dependent solubility difference and the common-ion effect, ammonium chloride is precipitated in a sodium chloride solution.
The Chinese name of Hou's process, lianhe zhijian fa (联合制碱法), means "Coupled Manufacturing Alkali Method": Hou's process is coupled to the Haber process and offers better atom economy by eliminating the production of calcium chloride since ammonia no longer needs to be regenerated. The byproduct ammonium chloride can be sold as a fertilizer.
See also
References
- ^ a b c d Harper, J.P (1936). Antipov, Evgeny; Bismayer, Ulrich; Huppertz, Hubert; Petrícek, Václav; Pöttgen, Rainer; Schmahl, Wolfgang; Tiekink, E.R.T.; Zou, Xiaodong (eds.). "Crystal Structure of Sodium Carbonate Monohydrate, Na2CO3. H2O". Zeitschrift für Kristallographie - Crystalline Materials. 95 (1). De Gruyter: 266–273. doi:10.1524/zkri.1936.95.1.266. ISSN 2196-7105. Retrieved 2014-07-25.
- ^ a b c d e f g Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. p. 633.
- ^ a b Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 208–209.
- ^ a b c d Anatolievich, Kiper Ruslan. "sodium carbonate". http://chemister.ru. Retrieved 2014-07-25.
{{cite web}}: External link in|website= - ^ a b c Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. p. 861. ISBN 0-07-049439-8.
- ^ a b c d Dusek, Michal; Chapuis, Gervais; Meyer, Mathias; Petricek, Vaclav (2003). "Sodium carbonate revisited" (PDF). Acta Crystallographica Section B. 59 (3). International Union of Crystallography: 337–352. doi:10.1107/S0108768103009017. ISSN 0108-7681. Retrieved 2014-07-25.
- ^ a b c Betzel, C.; Saenger, W.; Loewus, D. (1982). "Sodium Carbonate Heptahydrate". Acta Crystallographica Section B. 38 (11). International Union of Crystallography: 2802–2804. doi:10.1107/S0567740882009996.
- ^ a b c Sigma-Aldrich Co., Sodium carbonate. Retrieved on 2014-05-06.
- ^ http://chem.sis.nlm.nih.gov/chemidplus/rn/497-19-8
- ^ "Material Safety Data Sheet – Sodium Carbonate, Anhydrous" (PDF). http://www.conservationsupportsystems.com. ConservationSupportSystems. Retrieved 2014-07-25.
{{cite web}}: External link in|website= - ^ minerals.usgs.gov
- ^ Finishing techniques in Metalwork - Philadelphia Museum of Art
- ^ "Microcapsule material for carbon capture developed". February 6, 2015.
- ^ tatachemicals.com
- ^ a b Clow, Archibald and Clow, Nan L. (1952). Chemical Revolution, (Ayer Co Pub, June 1952), pp. 65–90. ISBN 0-8369-1909-2.
- ^ Kiefer, David M. (January 2002). "It was all about alkali". Today's Chemist at Work. 11 (1): 45–6.
Further reading
- Eggeman, T. (2011). "Sodium Carbonate". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1915040918012108.a01.pub3. ISBN 0471238961.
- Thieme, C. (2000). "Sodium Carbonates". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_299. ISBN 3527306730.
External links
- American Natural Soda Ash Company
- International Chemical Safety Card 1135
- FMC Wyoming Corporation
- Use of sodium carbonate in dyeing
- Sodium carbonate manufacturing by synthetic processes