Oxibendazole
Appearance
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.039.873 |
| Chemical and physical data | |
| Formula | C12H15N3O3 |
| Molar mass | 249.26 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Oxibendazole is a benzimidazole drug that is used to protect against roundworms, strongyles, threadworms, pinworms and lungworm infestations in horses and some domestic pets. It is usually white to yellowish in appearance, and may take the form of a powder or a tablet.
Synthesis
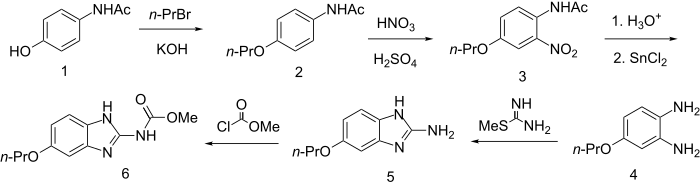
4-Hydroxyacetamide is alkylated with n-propylbromide in the presence of KOH to give the ether (2). Nitration of the product with HNO3/H2SO4 proceeds at the position ortho to the amido group (3); saponification followed by reduction of the nitro group with SnCl2gives the phenylenediamine (4). Then it is converted to the 2-aminobenzimidazole system by S-methylthiourea and subsequently acylated by methyl chloroformate to product 6.
References
