Hexazinone

| |
| Names | |
|---|---|
| IUPAC name
3-Cyclohexyl-6-dimethylamino-1-methyl-1,3,5-triazine-2,4-dione
| |
| Other names
Velpar
Hexazinone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.051.869 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20N4O2 | |
| Molar mass | 252.31 |
| Appearance | White crystalline solid |
| Density | 1.25 g/cm3 |
| Melting point | 116 °C (241 °F; 389 K) |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.[1]
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. Approximately 33% is used on alfalfa, 31% in forestry, 29% in industrial areas, 4% on rangeland and pastures, and < 2% on sugarcane.[2]
Hexazinone is a pervasive groundwater contaminant, due to its high water solubility
History
Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. It’s is used among a broad range of places. It is used to control weeds within all sort of applications. From sugarcane plantations, forestry field nurseries, pineapple plantations to high- and railway grasses and industrial plant sites. [3]
Hexazinone was first registered in 1975 for the overall control of weeds. registration for uses in crops were added later. For christmas and forage trees the use was registered in 1977. Also for the use on sugarcane use was registered in 1980 and for alfalfa in 1981.[4]
In 1982 and 1988 registration standards were issued by the EPA. These product summaries required further research for chemistry, toxicology, ecological effects and environmental fate data. [4]
In 1988 the office for drinking water from the EPA issued a drinking water Health Advisory (HA) for hexazinone.[4]
In 1989, hexazinone was deliberately used in an act of vandalism to poison the Treaty Oak in Austin, Texas.
In 1994 20 end-use pesticide products and one technical grade, manufacturing use product were registered which contained hexazinone.[4]
Structure and reactivity
Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells. [5]
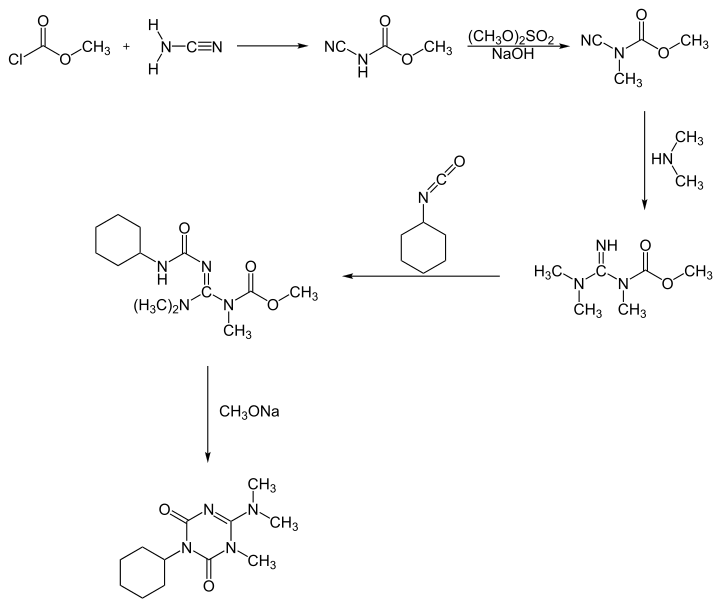
Synthesis
Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of methyl chloroformate with cyanamide, forming hexazinone after a five-step pathway[6]:
The second synthesis starts with methylthiourea which reacts with methyl bromide or methyl iodide[6]:
Volatilization
Although hexazinone does not volatilize rapidly in the field, the potential for volatilization increases with temperature, increasing soil moisture and decreasing clay and organic content.[7]
Photodegradation
Hexazinone degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the pH and the temperature of the water do not affect the photodegradation significantly.[8]
Microbial degradation
Hexazinone is mainly degraded by aerobic microorganisms in the soils. In soils which were kept in anaerobic conditions, no degradation occurred. However, in aerobic soils 45-75% of the applied hexazinone was released as CO2 as a result of microbial degradation.[9]
Adsorption
Adsorption of hexazinone to soil particles is low, but increases with increasing organic content, pH, and clay exchange capacity. The soil temperature however does not affect the adsorption significantly.[10]
Chemical decomposition
Hexazinone degrades into eight or more different metabolites: A through H.[8] Only metabolite B is toxic to plants, however it still has only 1% of the toxicity of hexazinone. The ratio between the different metabolites depends on the environmental conditions.[11]
Available forms
Mechanism of action
Hexazinone is a broad-spectrum residual and contact herbicide, rapidly absorbed by the leaves and roots. It is tolerated by conifers, and therefore it is a very effective herbicide for the control for annual and perennial broadleaf weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the herbicide to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.[12] It moves through the conductive tissues to the leaves, where it blocks the photosynthesis of the plant within the chloroplasts. Hexazinone binds to a protein of the photosystem II complex, which blocks the electron transport. The result are multiple following reactions. First triplet-state chlorophyll reacts with oxygen to form singlet oxygen. Both chlorophyll and singlet oxygen then remove hydrogen ions from the unsaturated lipids present in de cells and the organelle membranes, forming lipid radicals. These radicals will oxydize other lipids and proteins, eventually resulting in loss of the membrane integrity of the cells and organelles. This will result in a loss of chlorophyll, leakage of cellular contents, cell death, and eventually death of the plant.[13] Woody plants first show yellowing of the leaves before they start to defoliate, eventually they will die.[14] Sometimes plants are able to refoliate and defoliate again during the growing season.
Metabolism
Indications
Efficacy and side effects
Efficacy
adverse effects
Toxicity
Effects on animals
Risk assessment and regulations
References
- ^ Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165
- ^ Hexazinone, Herbicide Profile, Pesticide Management Education Program, Cornell University
- ^ Wang, Huili; Xu, Shuxia; Tan, Chengxia; Wang, Xuedong (2009-05-30). "Anaerobic biodegradation of hexazinone in four sediments". Journal of Hazardous Materials. 164 (2–3): 806–811. doi:10.1016/j.jhazmat.2008.08.073.
- ^ a b c d "Hexazinone: Reregistration Eligibility Decision (RED) Fact Sheet" (PDF).
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ "Agronomy 317 - Iowa State University". agron-www.agron.iastate.edu. Retrieved 2017-03-15.
- ^ a b Ullmann's agrochemicals. Wiley-VCH. 2007-01-01. ISBN 9783527316045. OCLC 470787466.
- ^ Helling, C. S.; Kearney, P. C.; Alexander, M. (1971). "Behavior of pesticides in soil". Adv. Agron. 23: 147–240.
- ^ a b Rhodes, R. C. (1980b). "Studies with 14C-labeled hexazinone in water and bluegill sunfish". J. Agric. Food Chem. 28: 306–310.
- ^ Rhodes, R. C. (1980a). "Soil Studies with 14C-labeled hexazinone". J. Agric. Food Chem. 28: 311–315.
- ^ Koskiene, W. C.; Stone, D. M.; Harris, A. R. (1996). "Sorption of hexazinone, sulfometuron methyl, and tebuthiuron on acid, low base saturated sands". Chemosphere. 32(9): 1681–1689.
- ^ Roy, D. N.; Konar, S. K.; Charles, D. A.; Feng, J. C.; Prasad, R.; Campbell, R. A. (1989). "Determination of persistence, movement, and degradation of hexazinone in selected Canadian boreal forest soils". J. Agric. Food Chem. 37: 443–447.
- ^ Ghassemi, M.; et al. (1981). Environmental fates and impacts of major forest use pesticides. Washington D.C. pp. 169–194.
{{cite book}}: Explicit use of et al. in:|first=(help)CS1 maint: location missing publisher (link) - ^ "Weed Science Society of America". wssa.net. Retrieved 2017-03-15.
- ^ Sidhu, S. S.; Feng., J. C. (1993). "Hexazinone and its metabolites in boreal forest vegetation". Weed Sci. 41: 281–287.
External links
- DuPont webpage on Velpar
- Hexazinone in the Pesticide Properties DataBase (PPDB)