Hexazinone

| |
| Names | |
|---|---|
| IUPAC name
3-Cyclohexyl-6-dimethylamino-1-methyl-1,3,5-triazine-2,4-dione
| |
| Other names
Velpar
Hexazinone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.051.869 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20N4O2 | |
| Molar mass | 252.31 |
| Appearance | White crystalline solid |
| Density | 1.25 g/cm3 |
| Melting point | 116 °C (241 °F; 389 K) |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.[1]
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. Approximately 33% is used on alfalfa, 31% in forestry, 29% in industrial areas, 4% on rangeland and pastures, and < 2% on sugarcane.[2]
Hexazinone is a pervasive groundwater contaminant, due to its high water solubility.
History
Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. it’s is used among a broad range of places. it is used to control weeds within all sort of applications. from sugarcane plantations, forestry field nurseries, pineapple plantations to high- and railway grasses and industrial plant sites.[3]
Hexazinone was first registered in 1975 for the overall control of weeds and later for uses in crops. For christmas and forage trees the use was registered in 1977 and for the use on sugarcane and alfalfa in 1980 and 1981 respectively.[4]
In 1982 and 1988 registration standards were issued by the EPA. These product summaries required further research for chemistry, toxicology, ecological effects and environmental fate data. In 1988 the office for drinking water from the EPA issued a drinking water Health Advisory (HA) for hexazinone.[4]
In 1989, hexazinone was deliberately used in an act of vandalism to poison the Treaty Oak in Austin, Texas.
Structure and reactivity
Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells.[5]
Synthesis
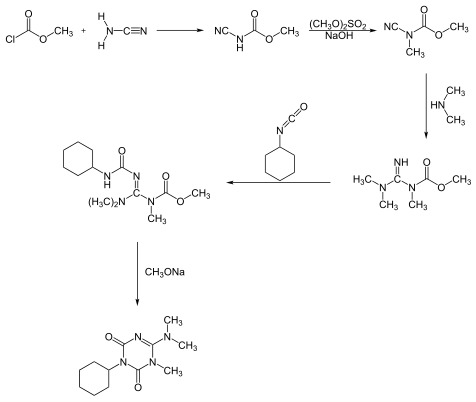
Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of methyl chloroformate with cyanamide, forming hexazinone after a five-step pathway:[6]
A second synthesis starts with methylthiourea which reacts with methyl bromide or methyl iodide:[6]
Degradation reactions
Volatilization
Although hexazinone does not volatilize rapidly in the field, the potential for volatilization increases with temperature, increasing soil moisture and decreasing clay and organic content.[7]
Photodegradation
Hexazinone degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the pH and the temperature of the water do not affect the photodegradation significantly.[8]
Microbial degradation
Hexazinone is mainly degraded by aerobic microorganisms in the soils. In soils which were kept in anaerobic conditions, no degradation occurred. However, in aerobic soils 45-75% of the applied hexazinone was released as CO2 as a result of microbial degradation.[9]
Adsorption
Adsorption of hexazinone to soil particles is low, but increases with increasing organic content, pH, and clay exchange capacity. The soil temperature however does not affect the adsorption significantly.[10]
Chemical decomposition
Hexazinone degrades into eight or more different metabolites: A through H. Only metabolite B is toxic to plants, however it still has only 1% of the toxicity of hexazinone.[8] The ratio between the different metabolites depends on the environmental conditions.[11]
Mechanism of action
Hexazinone is a broad-spectrum residual and contact herbicide, rapidly absorbed by the leaves and roots. It is tolerated by conifers, and therefore it is a very effective herbicide for the control for annual and perennial broadleaf weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the herbicide to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.[12] It moves through the conductive tissues to the leaves, where it blocks the photosynthesis of the plant within the chloroplasts. Hexazinone binds to a protein of the photosystem II complex, which blocks the electron transport. The result are multiple following reactions. First triplet-state chlorophyll reacts with oxygen to form singlet oxygen. Both chlorophyll and singlet oxygen then remove hydrogen ions from the unsaturated lipids present in de cells and the organelle membranes, forming lipid radicals. These radicals will oxydize other lipids and proteins, eventually resulting in loss of the membrane integrity of the cells and organelles. This will result in a loss of chlorophyll, leakage of cellular contents, cell death, and eventually death of the plant.[13] Woody plants first show yellowing of the leaves before they start to defoliate, eventually they will die.[14] Sometimes plants are able to refoliate and defoliate again during the growing season.
Metabolism
Hexazinone has seven naturally occurring metabolites A, B, C, D, E, G, and H. These metabolites occur through the degradation of hexazinone, plant metabolism being the leading factor since it is a herbicide. The major pathways of hexazinone degradation are photodegradation and biological decomposition.[15] In aqueous solution a slow degradation under light occurs into metabolite A by hydroxylation, and into metabolites B and H by demethylation.[16] Degradation into metabolites A, B, C, and D happens under the influence of microbes this process occurs more eagerly than photodegradation.[17] It is found that this degradation is primarily by hydroxylation and demethylation of the cyclohexyl ring.[18] Hexazinone can also be metabolised within multiple plant species, this may vary from study to study; nontheles degradation into metabolites A, B, C, D, and E have been observed in one or more of these studies.[19][20][21]
Indications
In a case report where a 26 year old woman inhaled an unknown amount of hexazinone dust it was found that the only occurrence of indication was vomiting within 24 hours. No other symptoms were reported and no further treatment was administered.[22]
Efficacy and adverse effects
Efficacy
Hexazinone is being used as a herbicide. It is absorbed from the soil and inhibits photosynthesis in plants. In this way, the growth of unwanted plants in controlled.[23] Because of the high water-solubility, hexazinone is very mobile in the soil.[24] It is also very persistent, and has a half-life between a couple of days[25] to more than nine months[26]. The effect of a single application of hexazinone can be observed for a long period.
The benefit of using hexazinone compared to mechanical weed control techniques is investigated. The application of hexazinone causes less changes in water quality than the mechanical techniques.[27]
Adverse effects
Because it is non-selective, not only the weed, but also the crop can be affected. The water-solubility and the persistence in water are not only advantages for the efficacy, but can also be disadvantages. It can move through the soil and can affect plants 100 meters away from the location where the chemical is administered.[28]
By moving through ground water, the hexazinone also affects the organisms living around the agriculture area. Organisms take in the chemical by drinking water or eating contaminated plants. For small mammals and aquatic and terrestrial plants the levels of hexazinone even exceed the LOC (levels of concern). It is also highly toxic for algae. This affects all the aquatic wildlife, because the aquatic plants and algae are the food and oxygen source for fish.[4]
Larch trees (Larix spp.) experience toxic effects when they take up hexazinone, so the herbicide cannot be applicated in region where these trees vegetate.[29]
Toxicity
Animal studies have shown that hexazinone causes acute, severe eye irritation. Therefore, it has been labelled as a Toxicity Category I compound for primary eye irritation, which is the highest classification for toxicity. There is a relatively low acute toxicity in case of ingestion (Toxicity Category III), inhalation or skin contact (Toxicity Category IV).[4]
The EPA has classified hexazinone as a Group D carcinogen. If a chemical is listed as a Group D, it means there is no evidence for carcinogenicity, but there is also no evidence that it is not carcinogenic. Thus, the results from studies do not accept nor reject the carcinogenicity.[4]
The ADI (acceptable daily intake) for hexazinone is 0.1 mg/kg/day and the NOAEL (No Observed Adverse Effect Level) is 10 mg/kg/day.[30]
People working in the agriculture sector do not have to wear additional protection, but should be careful during the application of hexazinone due to the acute eye toxicity.[4]
Effects on animals
To birds and mammals hexazinone has a low toxicity. The uptake from treated crops will not be enough to reach a toxic level in animals.[14] Beside the excretion in animals occurs rapidly whereby there is no accumulation in the organism.[29] The NOAEL in rats is 200 ppm and NOAEL in dogs is 1000 ppm.[31] To most fish and reptiles hexazinone is slightly toxic.[24] The LC50 values for rainbow trout and bluegill are respectively 320 mg/L and 370 mg/L.[29]
Because hexazinone can have a long half-life in water, the residues exceed the LOC. Consequently, a high toxicity arises for aquatic plants and algae. Therefore, the fish eating the algae and plants also undergo the toxic effects or die because of a lack of food. This can result in a collapse of the food chain, which influences the ecological environment.[32]
Risk assessment and regulations
Usage
Herbicides containing hexazinone should not be used on lawns, driveways, walks, tennis courts, and similar areas.[33] Drift of dry herbicide powder must be prevented, it should only be applied to the desired plants. Contamination of any body of water must also be prevented.
Disposal
The safest way to dispose of excess herbicides containing hexazinone is to, in case of small amounts use it. If usage of the remaining herbicide is not possible, dispose of it in a household hazardous waste collection program or a similar program for the elimination of unwanted, residual herbicides. Verify with your local solid waste management authority, health department, or environmental agency to find out where this is locally possible. Information about any local regulations for the waste disposal of herbicides can also be found at these authorities. Empty herbicide container should be regarded as full because they are residually contaminated. These containers should not be reused and disposed of following the directions on the container.
Allowable Tolerances
Established tolerances for combined residues containing hexazinone herbicides and its metabolites on food are as follows:
| Allowed residues | Food products |
|---|---|
| 0.1 ppm | Cattle fat, meat, meat by-products; goat fat, meat, meat by-products; hog fat, meat, meat by-products; horse fat, meat, meat by-products; sheep fat, meat, meat by-products; milk |
| 0.2 ppm | Blueberry |
| 0.5 ppm | Pineapple (whole fruit) |
| 2.0 ppm | Alfalfa green forage |
| 8.0 ppm | Alfalfa hay |
| 10 ppm | Grass pasture, range |
Hazards
The following health and environmental hazards apply to hexazinone herbicides:[34][35]
| Code | Applies to | Statement |
|---|---|---|
| H302 | Acute oral toxicity, category 4 | “Harmful if swallowed” |
| H319 | Severe eye damage/irritation, category 2A | “Causes serious eye irritation” |
| H400 | Acute danger for aquatic environment, category 1 | “Very toxic to aquatic life” |
| H410 | Chronic danger for aquatic environment, category 1 | “Very toxic to aquatic life with long-lasting effects” |
Precaution
The following prevention, response, and disposal precautionary statements apply to hexazinone herbicides:[36]
| Code | Statement |
|---|---|
| P261 | “Avoid breathing dust/fumes/gas/mist/vapours/spray” |
| P264 | “Wash … thoroughly after handling” |
| P270 | “Do not eat, drink, or smoke when using this product” |
| P273 | “Avoid release to the environment” |
| P280 | “Wear protective gloves/protective clothing/eye protection/face protection” |
| P301+P330+P312 | “IF SWALLOWED: Rinse mouth. Call a POISON CENTER/doctor if you feel unwell” |
| P304+P340 | “IF INHALED: Remove person to fresh air and keep comfortable for breathing” |
| P305+P351+P338 | “IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing” |
| P337+P313 | “If eye irritation persists: Get medical adviece/attention” |
| P391 | “Collect spillage” |
| P501 | “Dispose of contents/container to … [… in accordance with local/regional/national/international regulation (as specified under Disposal)]” |
References
- ^ Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165
- ^ Hexazinone, Herbicide Profile, Pesticide Management Education Program, Cornell University
- ^ Wang, Huili; Xu, Shuxia; Tan, Chengxia; Wang, Xuedong (2009-05-30). "Anaerobic biodegradation of hexazinone in four sediments". Journal of Hazardous Materials. 164 (2–3): 806–811. doi:10.1016/j.jhazmat.2008.08.073.
- ^ a b c d e f "Hexazinone: Reregistration Eligibility Decision (RED) Fact Sheet" (PDF).
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ "Agronomy 317 - Iowa State University". agron-www.agron.iastate.edu. Retrieved 2017-03-15.
- ^ a b Ullmann's agrochemicals. Wiley-VCH. 2007-01-01. ISBN 9783527316045. OCLC 470787466.
- ^ Helling, C. S.; Kearney, P. C.; Alexander, M. (1971). "Behavior of pesticides in soil". Adv. Agron. 23: 147–240.
- ^ a b Rhodes, R. C. (1980b). "Studies with 14C-labeled hexazinone in water and bluegill sunfish". J. Agric. Food Chem. 28: 306–310.
- ^ Rhodes, R. C. (1980a). "Soil Studies with 14C-labeled hexazinone". J. Agric. Food Chem. 28: 311–315.
- ^ Koskiene, W.C.; Stone, D.M.; Harris, A.R. (1996). "Sorption of hexazinone, sulfometuron methyl, and tebuthiuron on acid, low base saturated sands". Chemosphere. 32(9): 1681–1689.
- ^ Roy, D.N.; Konar, S.K.; Charles, D.A.; Feng, J.C.; Prasad, R.; Campbell, R.A. (1989). "Determination of persistence, movement, and degradation of hexazinone in selected Canadian boreal forest soils". J. Agric. Food Chem. 37: 443–447.
- ^ Ghassemi, M.; et al. (1981). Environmental fates and impacts of major forest use pesticides. Washington D.C. pp. 169–194.
{{cite book}}: Explicit use of et al. in:|first=(help)CS1 maint: location missing publisher (link) - ^ "Weed Science Society of America". wssa.net. Retrieved 2017-03-15.
- ^ a b Sidhu, S. S.; Feng., J. C. (1993). "Hexazinone and its metabolites in boreal forest vegetation". Weed Sci. 41: 281–287.
- ^ Ghassemi, M; et al. (1981). Environmental fates and impacts of major forest use pesticides. Washington D.C. pp. 169–194.
{{cite book}}: Explicit use of et al. in:|last2=(help)CS1 maint: location missing publisher (link) - ^ Rhodes, R.C. (1987). Decomposition of “Velpar” weed killer in soil.
- ^ Neary, D.G.; Bush, P.B.; Michael, J.L. (1993). Fate, dissipation and environmental effects of pesticides in southern forests: A review of a decade of research progress. J. of Environ. Toxicol. and Chem. p. 12:411-428.
- ^ Rhodes, R.C. (1980). Soil studies with 14C-labeled hexazinone. J. of Agric. and Food Chem. Vol. 28, No 2. p. 311-315.
- ^ Baron, J.L.; Monaco, T.J. (1986). Uptake, translocation, and metabolism of hexazinone in blueberry (Vaccinium spl.) and Hollow Goldenrod (Solidago fistulosa). Weed Science. pp. 34:824- 829.
- ^ Jensen, K.I.N.; Kimball, E.R. (1990). Uptake and metabolism of hexazinone in Rubus hispidus L. and Pyrus melanocarpa (Michx.) Willd. Weed Res. pp. 30: 35-41.
- ^ Sidhu, S.S.; Feng, J.C. (1993). Hexazinone and its metabolites in boreal forest vegetation. Weed Sci. pp. 41:281-287.
- ^ Environmental Protection Agency (1988). Health Advisories for 50 Pesticides. Simazine (USNTIS, PB 88-245931), Washington DC, EPA Office of Drinking Water. pp. 765–788.
- ^ "Haz-Map Category Details". hazmap.nlm.nih.gov. Retrieved 2017-03-17.
- ^ a b EXTOXNET 1996. "Pesticide Information Profiles". Extension Toxicology Network.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help)CS1 maint: numeric names: authors list (link) - ^ Solomon, K.R.; Bowhey, C.S.; Liber, K.; Stephenson, G.R. (1988). "Persistence of hexazinone (Velpar), triclopyr (Garlon), and 2,4-D in a Northern Ontario aquatic environment". J. Agric. Food Chem. 36: 1314–1318.
- ^ Thompson, D.G.; MacDonald, L.M.; Staznik, B. (1992). "Persistence of hexazinone and metsulfuron-methyl in a mixed-wood/boreal forest lake". J. Agric. Food Chem. 40: 1444–1449.
- ^ Neary, D.G.; Bush, P.B.; Grant, A. (1986). "Water quality of ephemeral forest streams after site preparation with the herbicide hexazinone". For. Ecol. Manage. 14: 23–40.
- ^ Allender, W.J. (1991). "Movement of bromacil and hexazinone in a municipal site". Bull. Environ. Contam. Toxicol. 46: 284–291.
- ^ a b c Kamrin, M.A. (1997). Pesticide profiles. Toxicity, Environmenal Impact, and Fate. New York,United States of America: Lewis Publishers. pp. 351–354.
- ^ "Barrage Herbicide, SDS0867" (PDF). Cropcare. Retrieved 17-03-2017.
{{cite web}}: Check date values in:|access-date=(help); Cite has empty unknown parameter:|dead-url=(help) - ^ Gerald, L.; Kennedy Jr., A. (1984). "Chronic toxicity, reproductive, and teratogenic studies of hexazinone". Fundamental and Applied Toxicology. 4: 980–971.
- ^ Peterson, H.G.; Boutin, C.; Martin, P.A.; Freemark, K.E.; Ruecker, N.J.; Moody, M.J. (1994). "Aquatic phytotoxicity of 23 pesticides applied at expected environmental concentrations". Aquat. Toxicol. 28: 275–292.
- ^ "Hexazinone". PubChem. Retrieved 16 March 2017.
- ^ "hexazinone (Ref: DPX A3674)". PPDB: Pesticide Properties DataBase. Retrieved 14 March 2017.
- ^ "Hexazinone". EU Pesticides database. Retrieved 14 March 2017.
- ^ "Hexazinone". PubChem. Retrieved 16 March 2017.
External links
- DuPont webpage on Velpar
- Hexazinone in the Pesticide Properties DataBase (PPDB)