Amniocentesis
| Amniocentesis | |
|---|---|
| ICD-9-CM | 75.1 |
| MeSH | D000649 |
| MedlinePlus | 003921 |
Amniocentesis (also referred to as amniotic fluid test or AFT) is a medical procedure[1] used in prenatal diagnosis of chromosomal abnormalities and fetal infections,[2] and also for sex determination, in which a small amount of amniotic fluid, which contains fetal tissues, is sampled from the amniotic sac surrounding a developing fetus, and then the fetal DNA is examined for genetic abnormalities. The most common reason to have an "amnio" is to determine whether a baby has certain genetic disorders or a chromosomal abnormality, such as Down syndrome. Amniocentesis (or another procedure, called chorionic villus sampling (CVS)) can diagnose these problems in the womb.[3] Amniocentesis is performed when a woman is between 14 and 16 weeks gestation. Women who choose to have this test are primarily those at increased risk for genetic and chromosomal problems, in part because the test is invasive and carries a small risk of miscarriage. This process can be used for prenatal sex discernment and hence this procedure has legal restrictions in some countries. Amniocentesis was first introduced by American obstetrician Fritz Friedrich Fuchs and Danish gastroenterologist Polv Riis in 1956 for fetal sex determination and up to mid 1970s amniocentesis were done 'blind‘. Doctors Jens Bang and Allen Northeved from Denmark were the first to report amniocentesis done with the guide of an ultrasound in 1972. Chorionic Villus Sampling (CVS) was first performed by Italian biologist Giuseppe Simoni in 1983. Now real-time ultrasound is used during all invasive procedures because it provides for the safety of the fetus and accuracy of results.
Procedure
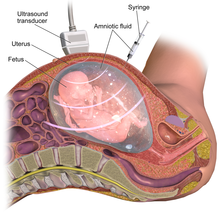
Before the start of the procedure, a local anesthetic can be given to the mother in order to relieve the pain felt during the insertion of the needle used to withdraw the fluid. After the local anesthetic is in effect, a needle is usually inserted through the mother's abdominal wall, then through the wall of the uterus, and finally into the amniotic sac. With the aid of ultrasound-guidance, a physician punctures the sac in an area away from the fetus and extracts approximately 20ml of amniotic fluid. If used for prenatal genetic diagnosis, fetal cells are separated from the extracted sample. The cells are grown in a culture medium, then fixed and stained. Under a microscope the chromosomes are examined for abnormalities. The most common abnormalities detected are Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Turner syndrome (monosomy X). In regard to the fetus, the puncture seals and the amniotic sac replenishes the liquid over the next 24–48 hours.
Indications and results
Genetic diagnosis
Early in pregnancy, amniocentesis is used for diagnosis of chromosomal and other fetal problems such as:[3]
- Down syndrome (trisomy 21)
- Trisomy 13
- Trisomy 18
- Fragile X
- Rare, inherited metabolic disorders
- Neural tube defects (anencephaly and spina bifida) by alpha-fetoprotein levels.[4]
Lung maturity
Amniocentesis can predict fetal lung maturity, which is inversely correlated to the risk of infant respiratory distress syndrome. In pregnancies of greater than 30 weeks, the fetal lung maturity may be tested by sampling the amount of surfactant in the amniotic fluid. Several tests are available that correlate with the production of surfactant. These include the lecithin-sphingomyelin ratio ("L/S ratio"), the presence of phosphatidylglycerol (PG), and more recently, the surfactant/albumin (S/A) ratio. For the L/S ratio, if the result is less than 2:1, the fetal lungs may be surfactant deficient. The presence of PG usually indicates fetal lung maturity. For the S/A ratio, the result is given as mg of surfactant per gm of protein. An S/A ratio <35 indicates immature lungs, between 35-55 is indeterminate, and >55 indicates mature surfactant production(correlates with an L/S ratio of 2.2 or greater).
Other
Amniocentesis can also be used to detect problems such as:
- Infection, in which amniocentesis can detect a decreased glucose level, a Gram stain showing bacteria or an abnormal differential count of white blood cells.[5]
- Rh incompatibility
- Decompression of polyhydramnios
An emerging indication for amniocentesis is in the management of preterm rupture of membranes where measurement of certain amniotic fluid inflammatory markers may be helpful. If amniotic fluid IL-6, a marker of inflammation, is elevated, the fetus is at high risk and delivery should be considered.[6]
Risks and drawbacks
Amniocentesis is performed between the 15th and 20th week of pregnancy; performing this test earlier may result in fetal injury.[7] The term "early amniocentesis" is sometimes used to describe use of the process between weeks 11 and 13.[8]
Complications of amniocentesis include preterm labor and delivery, respiratory distress, postural deformities, chorioamnionitis, fetal trauma and alloimmunisation of the mother (rhesus disease). Studies from the 1970s originally estimated the risk of amniocentesis-related miscarriage at around 1 in 200 (0.5%).[9] Three more recent studies from 2000-2006 estimated the procedure-related pregnancy loss at 0.6-0.86%.[10] A more recent study (2006) has indicated this may actually be much lower, perhaps as low as 1 in 1,600 (0.06%).[11] Unlike the previous studies, the number in this study only reflects the loss that resulted from amniocentesis complications and excluded the cases when parents decided for an abortion following the test results.[10] In contrast to amniocentesis, the risk of miscarriage from chorionic villus sampling (CVS) is believed to be approximately 1 in 100, although CVS may be done up to four weeks earlier, and may be preferable if the possibility of genetic defects is thought to be higher.[12]
Amniotic fluid embolism has been described as a possible risk.[13]
Social implications
The prenatal diagnosis of chromosomal abnormalities can have social drawbacks as technology changes the way people think about disability and kinship. There is potential for intensification of attitudes of discrimination towards those with a disability, whose births could have been prevented through technology such as amniocentesis. In one sense, amniocentesis offers a window of control and in another, an anxiety-provoking responsibility to make rational decisions about complex, emotional and culturally contingent issues.[14] [15]
Amniocentesis and stem cells
Recent studies have discovered that amniotic fluid can be a rich source of multipotent mesenchymal, hematopoietic, neural, epithelial, and endothelial stem cells.[16][17][18]
A potential benefit of using amniotic stem cells over those obtained from embryos is that they side-step ethical concerns among pro-life activists by obtaining pluripotent lines of undifferentiated cells without harm to a fetus or destruction of an embryo. These stem cells would also, if used to treat the same individual they came from, sidestep the donor/recipient issue which has so far stymied all attempts to use donor-derived stem cells in therapies.
Artificial heart valves, working tracheas, as well as muscle, fat, bone, heart, neural and liver cells have all been engineered through use of amniotic stem cells[citation needed]. Tissues obtained from amniotic cell lines show promise for patients suffering from congenital diseases/malformations of the heart, liver, lungs, kidneys, and cerebral tissue.[19]
The first amniotic stem cells bank in the US is active in Boston, Massachusetts.[20][21][22][23]
See also
- Chorionic villus sampling
- Percutaneous umbilical cord blood sampling
- Prenatal diagnosis
- Amniotic stem cells
- Amniotic fluid
References
- ^ The word amniocentesis itself indicates precisely the procedure in question, Gr. ἀμνίον amníon being the "inner membrane round the foetus" and κέντησις kéntēsis meaning "pricking", i.e. its puncture in order to retrieve some amniotic fluid.
- ^ "Diagnostic Tests – Amniocentesis". Harvard Medical School. Archived from the original on 2008-05-16. Retrieved 2008-07-15.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Carlson, Laura M.; Vora, Neeta L. (June 2017). "Prenatal Diagnosis: Screening and Diagnostic Tools". Obstetrics and Gynecology Clinics of North America. 44 (2): 245–256. doi:10.1016/j.ogc.2017.02.004. ISSN 1558-0474. PMC 5548328. PMID 28499534.
- ^ Dungan, Jeffrey S.; Elias, Sherman (November 2008). "Prenatal Diagnostic Testing". The Merck Manuals Online Medical Library. Archived from the original on 4 August 2010. Retrieved July 30, 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Medina, T. M.; Hill, D. A. (2006). "Preterm premature rupture of membranes: Diagnosis and management". American Family Physician. 73 (4): 659–664. PMID 16506709.
- ^ Kenyon, Anna P; Abi-Nader, Khalil N; Pandya, Pranav P (2010). "Pre-Term Pre-Labour Rupture of Membranes and the Role of Amniocentesis". Fetal and Maternal Medicine Review. 21 (2): 75–88. doi:10.1017/S096553951000001X.
- ^ Seeds, JW (August 2004). "Diagnostic mid trimester amniocentesis: how safe?". American Journal of Obstetrics and Gynecology. 191 (2): 607–15. doi:10.1016/j.ajog.2004.05.078. PMID 15343248.
- ^ Sundberg K; Bang J; Smidt-Jensen S; et al. (September 1997). "Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling". Lancet. 350 (9079): 697–703. doi:10.1016/S0140-6736(97)02449-5. PMID 9291904.
- ^ Amniocentesis Risk Overrated?. Webmd.com (2006-11-01). Retrieved on 2011-11-22.
- ^ a b [1]. Committee opinion, Society of Obstetricians and Gynaecologists of Canada.
- ^ Eddleman, Keith A.; Malone, Fergal D.; Sullivan, Lisa; Dukes, Kim; Berkowitz, Richard L.; Kharbutli, Yara; Porter, T Flint; Luthy, David A.; Comstock, Christine H. (2006). "Pregnancy loss rates after midtrimester amniocentesis". Obstet Gynecol. 108 (5): 1067–72. doi:10.1097/01.AOG.0000240135.13594.07. PMID 17077226.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Rhoads, George G.; Jackson, Laird G.; Schlesselman, Sarah E.; De La Cruz, Felix F.; Desnick, Robert J.; Golbus, Mitchell S.; Ledbetter, David H.; Lubs, Herbert A.; Mahoney, Maurice J. (1989). "The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities". New England Journal of Medicine. 320 (10): 609–17. doi:10.1056/NEJM198903093201001. PMID 2645520.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Dodgson J, Martin J, Boswell J, Goodall HB, Smith R (May 1987). "Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion". Br Med J (Clin Res Ed). 294 (6583): 1322–3. doi:10.1136/bmj.294.6583.1322. PMC 1246486. PMID 3109636.
- ^ Lock, M & Nguyen, V 2010, An Anthropology of Biomedicine, Wiley-Blackwell, Oxford.
- ^ Rapp, R 1998, ‘Refusing Prenatal Diagnosis: The Meanings of Bioscience in a Multicultural World’, Science, Technology, & Human Values, vol. 23, no.1. pp. 45-70.
- ^ Weiss, Rick (2007-01-08). "Scientists See Potential In Amniotic Stem Cells". The Washington Post. Retrieved 2010-04-23.
- ^ De Coppi P, Bartsch G, Siddiqui MM, et al. (January 2007). "Isolation of amniotic stem cell lines with potential for therapy". Nat. Biotechnol. 25 (1): 100–6. doi:10.1038/nbt1274. PMID 17206138.
- ^ "Stem Cells – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Stem cells scientific updates – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "European Biotech Company Biocell Center Opens First united state Facility for Preservation of Amniotic Stem Cells in Medford, Massachusetts | Reuters". 2009-10-22. Archived from the original on October 30, 2009. Retrieved 2010-01-11.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Europe's Biocell Center opens Medford office – Daily Business Update – The Boston Globe". 2009-10-22. Archived from the original on 12 January 2010. Retrieved 2010-01-11.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "The Ticker - BostonHerald.com". Retrieved 2010-01-11.
- ^ "Biocell partner with largest New England's hospital group to preserve amniotic stem cell". Archived from the original on 14 March 2010. Retrieved 2010-03-10.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
External links
- Amniodex is an interactive decision support intervention designed for women faced with the decision of whether to undergo amniocentesis.
- The Amniocentesis Report A Decision Guide for Expectant Parents and Health Care Professionals.
- http://www.sutherlandhealth.com/companies/condomania/product-range/vision/amniotic-leak-detector
