Troponin

Troponin, or the troponin complex, is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) that is integral to muscle contraction[2] in skeletal muscle and cardiac muscle, but not smooth muscle .
Discussions of troponin often pertain to its functional characteristics[citation needed] and/or to its usefulness as a diagnostic marker[citation needed] or therapeutic target[3] for various heart disorders in particular as a highly specific marker for myocardial infarction or heart muscle cell death.
Function
Troponin is attached to the protein tropomyosin and lies within the groove between actin filaments in muscle tissue. In a relaxed muscle, tropomyosin blocks the attachment site for the myosin crossbridge, thus preventing contraction. When the muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm. Some of this calcium attaches to troponin, which causes it to change shape, exposing binding sites for myosin (active sites) on the actin filaments. Myosin's binding to actin causes crossbridge formation, and contraction of the muscle begins.
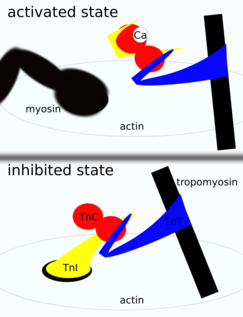
Troponin is found in both skeletal muscle and cardiac muscle, but the specific versions of troponin differ between types of muscle. The main difference is that the TnC subunit of troponin in skeletal muscle has four calcium ion-binding sites, whereas in cardiac muscle there are only three. Views on the actual amount of calcium that binds to troponin vary from expert to expert and source to source[citation needed].
Physiology
In both cardiac and skeletal muscles, muscular force production is controlled primarily by changes in the intracellular calcium concentration. In general, when calcium rises, the muscles contract and, when calcium falls, the muscles relax.
Troponin is a component of thin filaments (along with actin and tropomyosin), and is the protein complex to which calcium binds to trigger the production of muscular force. Troponin itself has three subunits, TnC, TnI, and TnT, each playing a role in force regulation [citation needed]. Under resting intracellular levels of calcium, tropomyosin covers the active sites on actin to which myosin (a molecular motor organized in muscle thick filaments) binds in order to generate force. When calcium becomes bound to specific sites in the N-domain of TnC, a series of protein structural changes occurs[citation needed] such that tropomyosin is rolled away from myosin-binding sites on actin, allowing myosin to attach to the thin filament and produce force and/or shorten the sarcomere.
Troponin I has also been shown to inhibit angiogenesis in vivo and in vitro.[4]
Individual subunits serve different functions:
- Troponin C binds to calcium ions to produce a conformational change in TnI
- Troponin T binds to tropomyosin, interlocking them to form a troponin-tropomyosin complex
- Troponin I binds to actin in thin myofilaments to hold the troponin-tropomyosin complex in place
Smooth muscle does not have troponin.[5]
Subunits
TnT is a tropomyosin-binding subunit which regulates the interaction of troponin complex with thin filaments; TnI inhibits ATP-ase activity of acto-myosin; TnC is a Ca2+-binding subunit, playing the main role in Ca2+ dependent regulation of muscle contraction.[6]
TnT and TnI in cardiac muscle are presented by forms different from those in skeletal muscles. Two isoforms of TnI and two isoforms of TnT are expressed in human skeletal muscle tissue (skTnI and skTnT). Only one tissue-specific isoform of TnI is described for cardiac muscle tissue (cTnI), whereas the existence of several cardiac specific isoforms of TnT (cTnT) are described in the literature. No cardiac specific isoforms are known for human TnC. TnC in human cardiac muscle tissue is presented by an isoform typical for slow skeletal muscle. Another form of TnC, fast skeletal TnC isoform, is more typical for fast skeletal muscles.[7] cTnI is expressed only in myocardium. No examples of cTnI expression in healthy or injured skeletal muscle or in other tissue types are known. cTnT is probably less cardiac specific. Expression of cTnT in skeletal tissue of patients with chronic skeletal muscle injuries has been described.[8]
Inside the cardiac troponin complex the strongest interaction between molecules has been demonstrated for cTnI – TnC binary complex especially in the presence of Ca2+ ( KA = 1.5x10−8 M−1).[9] TnC, forming a complex with cTnI, changes the conformation of cTnI molecule and shields part of its surface. According to the latest data cTnI is released in the blood stream of the patient in the form of binary complex with TnC or ternary complex with cTnT and TnC.[10] cTnI-TnC complex formation plays an important positive role in improving the stability of cTnI molecule. cTnI, which is extremely unstable in its free form, demonstrates significantly better stability in complex with TnC or in ternary cTnI-cTnT-TnC complex. It has been demonstrated that stability of cTnI in native complex is significantly better than stability of the purified form of the protein or the stability of cTnI in artificial troponin complexes combined from purified proteins.
Relation with contractile function and heart failure
Mutations in the cardiac troponin subunits can result in cardiomyopathies, including familial hypertrophic cardiomyopathy.[11]
Diagnostic use
An increased level of the cardiac protein isoform of troponin circulating in the blood has been shown to be a biomarker of heart disorders, the most important of which is myocardial infarction.[12] Raised troponin levels indicate cardiac muscle cell death as the molecule is released into the blood upon injury to the heart.
Cardiac conditions
Certain subtypes of troponin (cardiac I and T) are very sensitive and specific indicators of damage to the heart muscle (myocardium). They are measured in the blood to differentiate between unstable angina and myocardial infarction (heart attack) in people with chest pain or acute coronary syndrome. A person who recently had a myocardial infarction would have an area of damaged heart muscle and elevated cardiac troponin levels in the blood.[13] This can also occur in people with coronary vasospasm, a type of myocardial infarction involving severe constriction of the cardiac blood vessels. After a myocardial infarction troponins may remain high for up to 2 weeks.[14]
Cardiac troponins are a marker of all heart muscle damage, not just myocardial infarction, which is the most severe form of heart disorder. However, diagnostic criteria for raised troponin indicating myocardial infarction is currently set by the WHO at a threshold of 2 μg or higher. Critical levels of other cardiac biomarkers are also relevant, such as creatine kinase. Other conditions that directly or indirectly lead to heart muscle damage and death can also increase troponin levels, such as renal failure.[15][16] Severe tachycardia (for example due to supraventricular tachycardia) in an individual with normal coronary arteries can also lead to increased troponins for example, it is presumed due to increased oxygen demand and inadequate supply to the heart muscle.
Troponins are also increased in patients with heart failure, where they also predict mortality and ventricular rhythm abnormalities. They can rise in inflammatory conditions such as myocarditis and pericarditis with heart muscle involvement (which is then termed myopericarditis). Troponins can also indicate several forms of cardiomyopathy, such as dilated cardiomyopathy, hypertrophic cardiomyopathy or (left) ventricular hypertrophy, peripartum cardiomyopathy, Takotsubo cardiomyopathy, or infiltrative disorders such as cardiac amyloidosis.
Heart injury with increased troponins also occurs in cardiac contusion, defibrillation and internal or external cardioversion. Troponins are commonly increased in several procedures such as cardiac surgery and heart transplantation, closure of atrial septal defects, percutaneous coronary intervention, or radiofrequency ablation.
Non-cardiac conditions
The distinction between cardiac and non-cardiac conditions is somewhat artificial; the conditions listed below are not primary heart diseases, but they exert indirect effects on the heart muscle.
Troponins are increased in around 40% of patients with critical illnesses such as sepsis. There is an increased risk of mortality and length of stay in the intensive-care unit in these patients.[17] In severe gastrointestinal bleeding, there can also be a mismatch between oxygen demand and supply of the myocardium.
Chemotherapy agents can exert toxic effects on the heart (examples include anthracycline, cyclophosphamide, 5-fluorouracil, and cisplatin). Several toxins and venoms can also lead to heart muscle injury (scorpion venom, snake venom, and venom from jellyfish and centipedes). Carbon monoxide poisoning or cyanide poisoning can also be accompanied by release of troponins due to hypoxic cardiotoxic effects. Cardiac injury occurs in about one-third of severe CO poisoning cases, and troponin screening is appropriate in these patients.[18][19]
Some patients with dissection of the ascending aorta have elevated troponins, and increased hemodynamic stress has been suggested as a mechanism.[20]
In both primary pulmonary hypertension, pulmonary embolism, and acute exacerbations of chronic obstructive pulmonary disease (COPD), right ventricular strain with increased wall tension and ischemia. Of course, patients with COPD exacerbations might also have concurrent myocardial infarction or pulmonary embolism, so care has to be taken to attribute increased troponin levels to COPD.
Central nervous system disorders can lead to increased sympathetic tone and/or catecholamine release, which lead to cardiac overstimulation. This is seen in subarachnoid hemorrhage, stroke, intracranial hemorrhage, and (generalized) seizures (in patients with epilepsy or eclampsia, for example).
Patients with end-stage renal disease can have chronically elevated troponin T levels, which are linked to a poorer prognosis.[21][22] Troponin I is less likely to be falsely elevated.[21]
Strenuous endurance exercise such as marathons or triathlons can lead to increased troponin levels in up to one-third of subjects, but it is not linked to adverse health effects in these competitors.[23][24][25] High troponin T levels have also been reported in patients with inflammatory muscle diseases such as polymyositis or dermatomyositis.[26][27] Troponins are also increased in rhabdomyolysis.
In hypertensive disorders of pregnancy such as preeclampsia, elevated troponin levels indicate some degree of myofibrillary damage.[28][29]
Cardiac troponin T and I can be used to monitor drug and toxin-induced cardiomyocyte toxicity. .[30]
Prognostic use
Raised troponin levels are prognostically important in many of the conditions in which they are used for diagnosis.[31]
In a community-based cohort study indicating the importance of silent cardiac damage, troponin I has been shown to predict mortality and first coronary heart disease event in men free from cardiovascular disease at baseline.[32]
Subunits
First cTnI.[33] and later cTnT [34] were used as markers of cardiac cell death. Now both proteins are widely used for the diagnosis of acute myocardial infarction (AMI), unstable angina, post-surgery myocardium trauma and some other diseases related with cardiac muscle injury. Both markers can be detected in patient’s blood 3–6 hours after onset of the chest pain, reaching peak level within 16–30 hours. Elevated concentration of cTnI and cTnT in blood samples can be detected even 5–8 days after onset of the symptoms, making both proteins useful also for the late diagnosis of AMI.[35]
Detection
Cardiac troponin T and I are measured by immunoassay methods.[36][37]
- Due to patent regulations, a single manufacturer (Roche Diagnostics) distributes cTnT.
- A host of diagnostic companies make cTnI immunoassay methods available on many different immunoassay platforms.[37]
Troponin elevation following cardiac cell necrosis starts within 2–3 hours, peaks in approx. 24 hours, and persists for 1–2 weeks.[38]
See also
References
- ^ PDB: 1J1E; Takeda S, Yamashita A, Maeda K, Maeda Y (2003). "Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form". Nature. 424 (6944): 35–41. doi:10.1038/nature01780. PMID 12840750.; rendered with PyMOL
- ^ "troponin" at Dorland's Medical Dictionary
- ^ "A structural and functional perspective into the mechanism of Ca2+-sensitizers that target the cardiac troponin complex". Journal of Molecular and Cellular Cardiology. 49 (6): 1031–1041. 2010. doi:10.1016/j.yjmcc.2010.08.019. PMC 2975748. PMID 20801130.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Moses, Marsha A.; Wiederschain D.; Wu I.; Fernandez, C.; Ghazizadeh V.; Lane W.; Flynn E.; Sytkowski A.; Tao T.; Langer R. (1999). "Troponin I is present in human cartilage and inhibits angiogenesis". Proceedings of the National Academy of Sciences of the United States of America. 96 (6): 2645–2650. Bibcode:1999PNAS...96.2645M. doi:10.1073/pnas.96.6.2645. JSTOR 47416. PMC 15822. PMID 10077564.
- ^ Troponins at eMedicine
- ^ Gomes, A.V; Potter, J.D.; Szczesna-Cordary, D. (2002). "The role of Troponin in muscle contraction". Life (54): 323–333.
- ^ Marston, S.B.; Redwood, C.S. (2003). "Modulation of thin filament activation by breakdown or isoform switching of thin filament Proteins". Circ. Res. 93 (12): 1170–1178. doi:10.1161/01.RES.0000105088.06696.17. PMID 14670832.
- ^ Sarko J, Pollack CV Jr (2002). "Cardiac troponins". J Emerg Med. 23 (1): 57–65. doi:10.1016/S0736-4679(02)00463-8. PMID 12217473.
- ^ Reiffert SU, Jaquet K, Heilmeyer LM Jr, Herberg FW (1998). "Stepwise subunit interaction changes by mono- and bisphosphorylation of cardiac troponin I". Biochemistry. 37 (39): 13516–13525. doi:10.1021/bi980280j. PMID 9753437.
- ^ Katrukha AG, Bereznikova AV, Esakova TV, Pettersson K, Lovgren T, Severina ME, Pulkki K, Vuopio-Pulkki LM, Gusev NB (1997). "Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex". Clin. Chem. 43 (8): 1379–1385. PMID 9267317.
- ^ Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD (May 2010). "Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function?". J. Mol. Cell. Cardiol. 48 (5): 882–92. doi:10.1016/j.yjmcc.2009.10.031. PMID 19914256.
- ^ Myocardial Infarction Workup. Archived 2016-06-09 at the Wayback Machine
- ^ Antman EM, Tanasijevic MJ, Thompson B, et al. (October 1996). "Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes". N. Engl. J. Med. 335 (18): 1342–9. doi:10.1056/NEJM199610313351802. PMID 8857017.
- ^ Amsterdam, E. A.; Wenger, N. K.; Brindis, R. G.; Casey, D. E.; Ganiats, T. G.; Holmes, D. R.; Jaffe, A. S.; Jneid, H.; Kelly, R. F.; Kontos, M. C.; Levine, G. N.; Liebson, P. R.; Mukherjee, D.; Peterson, E. D.; Sabatine, M. S.; Smalling, R. W.; Zieman, S. J. (23 September 2014). "2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". Circulation. 130: e344–e426. doi:10.1161/CIR.0000000000000134. PMID 25249585.
- ^ Ammann P, Pfisterer M, Fehr T, Rickli H (May 2004). "Raised cardiac troponins". BMJ. 328 (7447): 1028–9. doi:10.1136/bmj.328.7447.1028. PMC 403831. PMID 15117768.
- ^ Tsai SH, Chu SJ, Hsu CW, Cheng SM, Yang SP (March 2008). "Use and interpretation of cardiac troponins in the ED". Am J Emerg Med. 26 (3): 331–41. doi:10.1016/j.ajem.2007.05.031. PMID 18358946.
- ^ Lim W, Qushmaq I, Devereaux PJ, et al. (2006). "Elevated cardiac troponin measurements in critically ill patients". Arch. Intern. Med. 166 (22): 2446–54. doi:10.1001/archinte.166.22.2446. PMID 17159009.
- ^ Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD (January 2006). "Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning". JAMA. 295 (4): 398–402. doi:10.1001/jama.295.4.398. PMID 16434630.
- ^ Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD (May 2005). "Cardiovascular manifestations of moderate to severe carbon monoxide poisoning". J. Am. Coll. Cardiol. 45 (9): 1513–6. doi:10.1016/j.jacc.2005.01.044. PMID 15862427.
- ^ Bonnefoy E, Godon P, Kirkorian G, Chabaud S, Touboul P (April 2005). "Significance of serum troponin I elevation in patients with acute aortic dissection of the ascending aorta". Acta Cardiol. 60 (2): 165–70. doi:10.2143/AC.60.2.2005027. PMID 15887472.
- ^ a b Needham DM, Shufelt KA, Tomlinson G, Scholey JW, Newton GE (October 2004). "Troponin I and T levels in renal failure patients without acute coronary syndrome: a systematic review of the literature". Can J Cardiol. 20 (12): 1212–8. PMID 15494773.
- ^ Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A (November 2005). "Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis". Circulation. 112 (20): 3088–96. doi:10.1161/CIRCULATIONAHA.105.560128. PMID 16286604.
- ^ Rifai N, Douglas PS, O'Toole M, Rimm E, Ginsburg GS (April 1999). "Cardiac troponin T and I, echocardiographic [correction of electrocardiographic] wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon". Am. J. Cardiol. 83 (7): 1085–9. doi:10.1016/S0002-9149(99)00020-X. PMID 10190525.
- ^ Neumayr G, Gaenzer H, Pfister R, et al. (February 2001). "Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise". Am. J. Cardiol. 87 (3): 369–71, A10. doi:10.1016/S0002-9149(00)01382-5. PMID 11165984.
- ^ Urhausen A, Scharhag J, Herrmann M, Kindermann W (September 2004). "Clinical significance of increased cardiac troponins T and I in participants of ultra-endurance events". Am. J. Cardiol. 94 (5): 696–8. doi:10.1016/j.amjcard.2004.05.050. PMID 15342317.
- ^ Kobayashi S, Tanaka M, Tamura N, Hashimoto H, Hirose S (September 1992). "Serum cardiac troponin T in polymyositis/dermatomyositis". Lancet. 340 (8821): 726. doi:10.1016/0140-6736(92)92262-E. PMID 1355820.
- ^ Erlacher P, Lercher A, Falkensammer J, et al. (April 2001). "Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis". Clin. Chim. Acta. 306 (1–2): 27–33. doi:10.1016/S0009-8981(01)00392-8. PMID 11282091.
- ^ Fleming SM, O'Gorman T, Finn J, Grimes H, Daly K, Morrison JJ (November 2000). "Cardiac troponin I in pre-eclampsia and gestational hypertension". BJOG. 107 (11): 1417–20. doi:10.1111/j.1471-0528.2000.tb11658.x. PMID 11117772.
- ^ Morton A (July 2004). "Raised cardiac troponins: troponin is raised in pre-eclampsia". BMJ. 329 (7457): 111. doi:10.1136/bmj.329.7457.111-a. PMC 449874. PMID 15242925.
- ^ Gaze DC, Collinson PO; Collinson, PO (December 2005). "Cardiac troponins as biomarkers of drug- and toxin-induced cardiac toxicity and cardioprotection". Expert Opin Drug Metab Toxicol. 1 (4): 715–25. doi:10.1517/17425255.1.4.715. PMID 16863435.
- ^ Mannu, GS (August 2014). "The non-cardiac use and significance of cardiac troponins". Scottish medical journal. 59 (3): 172–8. doi:10.1177/0036933014540090. PMID 24934496.
- ^ Zethelius B, Johnston N, Venge P (February 2006). "Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study". Circulation. 113 (8): 1071–8. doi:10.1161/CIRCULATIONAHA.105.570762. PMID 16490824.
- ^ Cummins B, Auckland ML, Cummins P (1987). "Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction". Am Heart J. 113 (6): 1333–1344. doi:10.1016/0002-8703(87)90645-4. PMID 3591601.
- ^ Katus HA, Remppis A, Looser S, Hallermeier K, Scheffold T, Kubler W (1989). "Enzyme linked immunoassay of cardiac troponin T for the detection of acutemyocardial infarction in patients". J Moll Cell Cardiol. 21 (12): 1349–1353. doi:10.1016/0022-2828(89)90680-9.
- ^ Hamm CW. (2001). "Acute coronary syndromes. The diagnostic role of troponins". Thromb Res. 103 (1): 63–69. doi:10.1016/S0049-3848(01)00299-7.
- ^ Melanson SE, Tanasijevic MJ, Jarolim P (October 2007). "Cardiac troponin assays: a view from the clinical chemistry laboratory". Circulation. 116 (18): e501–4. doi:10.1161/CIRCULATIONAHA.107.722975. PMID 17967982.
- ^ a b Collinson PO, Boa FG, Gaze DC (September 2001). "Measurement of cardiac troponins". Ann. Clin. Biochem. 38 (Pt 5): 423–49. doi:10.1258/0004563011901109. PMID 11587122.
- ^ Patil, H.; Vaidya, O.; Bogart, D. (2011). "A Review of Causes and Systemic Approach to Cardiac Troponin Elevation". Clin Cardiol. 34: 723–728. doi:10.1002/clc.20983.
External links
- Troponin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Troponins at Lab Tests Online
