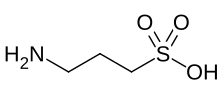
Homotaurine
| |
| |
| Names | |
|---|---|
| IUPAC name
3-Aminopropane-1-sulfonic acid
| |
| Other names
Tramiprosate; Alzhemed; 3-APS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.889 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO3S | |
| Molar mass | 139.17 g·mol−1 |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homotaurine (3-amino-1-propanesulfonic acid (3-APS) or tramiprosate (INN)) is a natural organic compound found in seaweed.[2] It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.[3]
In a phase 2 study Homotaurine reduced Abeta(42) levels a type of amyloid beta (Abeta), a toxic protein known to aggregate, leading to amyloid plaque deposition in the brain.[4]
In 2012 Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease that did not show efficacy in its primary endpoints but did show positive and significant effects of homotaurine on secondary endpoints and subgroups of patients, including a reduction in hippocampal volume loss and lower decline in memory function in the overall cohort, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting a disease-modifying effects.[5]
October 24, 2017 The FDA Granted Fast Track Designation to Alzheon’s ALZ-801 Development Program for the Treatment of Alzheimer’s Disease.[6]
In 2018 a new phase 3 study for genetically-defined subpopulation of high risk patients who are homozygous for the ε4 allele of apolipoprotein E (APOE4/4 homozygotes) at the Mild stage of AD has been filed with the FDA under the name ALZ-801 by the drug company Alzheon.[7][8]
It is approved by Canada's FDA to sell as a dietary supplement.[9]
It is approved in Israel as a dietary supplement.[10]
Biochemical properties
In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates.[5][11] Homotaurine has also shown anticonvulsant activities, reduction in skeletal muscle tonus, and hypothermic activity.[12]
Homotaurine has been reported as a GABA antagonist[3] as well as a GABA agonist.[12][13] In vitro studies have found that homotaurine is a GABAA partial agonist[14] as well as a GABAB receptor partial agonist with low efficacy, becoming an antagonist and a displacing full agonist of GABA or baclofen at this receptor.[15] In a study in rats, homotaurine reversed the catatonia induced by baclofen (the prototypical GABAB agonist),[16] and was able to produce analgesia via the GABAB receptor, an effect that was abolished when CGP 35348, a GABAB receptor antagonist was applied.[17][18]
One study suggests Homotaurine increases dopamine levels.[19]
One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to the N-acetyl derivative of homotaurine, acamprosate.[20] Acamprosate was approved by the FDA in 2004 to treat alcohol dependence.[3]
Homotaurine has shown pain relieving effects in animal studies that may involve the opioid receptors.[17]
See also
- Acamprosate – a derivative of homotaurine
- Teprosilic acid
References
- ^ Homotaurine at Sigma-Aldrich
- ^ https://patents.google.com/patent/WO2010096925A1/en[full citation needed]
- ^ a b c Lednicer, Daniel (2008). The Organic Chemistry of Drug Synthesis (7th ed.). Hoboken: John Wiley & Sons. p. 15. ISBN 978-0-470-18066-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Aisen, P. S; Saumier, D; Briand, R; Laurin, J; Gervais, F; Tremblay, P; Garceau, D (2006). "A Phase II study targeting amyloid- with 3APS in mild-to-moderate Alzheimer disease". Neurology. 67 (10): 1757–63. doi:10.1212/01.wnl.0000244346.08950.64. PMID 17082468.
- ^ a b Caltagirone, C; Ferrannini, L; Marchionni, N; Nappi, G; Scapagnini, G; Trabucchi, M (2012). "The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: A review". Aging clinical and experimental research. 24 (6): 580–7. doi:10.3275/8585. PMID 22961121.
- ^ https://alzheon.com/fda-grants-fast-track-designation-to-alzheons-alz-801-development-program-for-the-treatment-of-alzheimers-disease/[full citation needed]
- ^ https://alzheon.com/[full citation needed]
- ^ https://www.iadvanceseniorcare.com/article/alzheon-goes-public-fund-alzheimers-drug[full citation needed]
- ^ http://www.canadadrugcenter.com/Health-Canada-Approves-VIVIMIND.asp[full citation needed]
- ^ http://www.levpharm.com/site/flash/flashDetail.asp?flash_id=1124719[full citation needed]
- ^ Aisen, Paul; Gauthier, Serge; Vellas, Bruno; Briand, Richard; Saumier, Daniel; Laurin, Julie; Garceau, Denis (2007). "Alzhemed: A Potential Treatment for Alzheimers Disease". Current Alzheimer Research. 4 (4): 473–8. doi:10.2174/156720507781788882. PMID 17908052.
- ^ a b Oja SS and Kontro P. Taurine. Chapter 18 in Metabolism in the Nervous System, Ed. Lajtha ANS. Springer Science & Business Media, 2013. ISBN 9781468443677. Page 520
- ^ Armen H. Tashjian and Ehrin J. Armstrong. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Edited by David E. Golan. Lippincott Williams & Wilkins, 2011 ISBN 9781451118056. Page 308
- ^ Reyes-Haro, Daniel; Cabrera-Ruíz, Elizabeth; Estrada-Mondragón, Argel; Miledi, Ricardo; Martínez-Torres, Ataúlfo (2014). "Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs". Amino Acids. 46 (11): 2587–93. doi:10.1007/s00726-014-1813-0. PMID 25119985.
- ^ Giotti, A; Luzzi, S; Spagnesi, S; Zilletti, Lucilla (1983). "Homotaurine: A GABAB antagonist in guinea-pig ileum". British Journal of Pharmacology. 79 (4): 855–62. doi:10.1111/j.1476-5381.1983.tb10529.x. PMC 2044932. PMID 6652358.
- ^ Mehta, A; Ticku, M (1987). "Baclofen induces catatonia in rats". Neuropharmacology. 26 (9): 1419–23. doi:10.1016/0028-3908(87)90108-0. PMID 2823166.
- ^ a b Serrano, M.Isabel; Serrano, Jose S; Fernández, Ana; Asadi, Ihklas; Serrano-Martino, M.Carmen (1998). "GABAB Receptors and Opioid Mechanisms Involved in Homotaurine-Induced Analgesia". General Pharmacology: the Vascular System. 30 (3): 411–5. doi:10.1016/s0306-3623(97)00279-6. PMID 9510095.
- ^ Serrano, Maria Isabel; Serrano, Jose S; Asadi, Ikhlas; Fernandez, Ana; Serrano-Martino, Maria Carmen (2001). "Role of K+-channels in homotaurine-induced analgesia". Fundamental and Clinical Pharmacology. 15 (3): 167–73. doi:10.1046/j.1472-8206.2001.00026.x. PMID 11468027.
- ^ Ruotsalainen, M; Majasaari, M; Salimäki, J; Ahtee, L (1998). "Locally infused taurine, GABA and homotaurine alter differently the striatal extracellular concentrations of dopamine and its metabolites in rats". Amino Acids. 15 (1–2): 117–34. doi:10.1007/BF01345285. PMID 9871492.
- ^ Olive, M.Foster; Nannini, Michelle A; Ou, Christine J; Koenig, Heather N; Hodge, Clyde W (2002). "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". European Journal of Pharmacology. 437 (1–2): 55–61. doi:10.1016/s0014-2999(02)01272-4. PMID 11864639.
