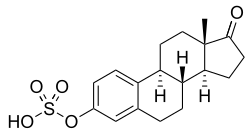
Estrone sulfate
| |
| |
| Names | |
|---|---|
| IUPAC name
[(8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate
| |
| Other names
E1S; Oestrone sulfate; Estrone 3-sulfate; Estra-1,3,5(10)-trien-17-one 3-sulfate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.888 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H22O5S | |
| Molar mass | 350.429 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Estrone sulfate (E1S), or estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.[1][2][3]
In addition to its role as a natural hormone, estrone sulfate is used as a medication, for instance in menopausal hormone therapy; for information on estrone sulfate as a medication, see the estrone sulfate (medication) article.
Biological function
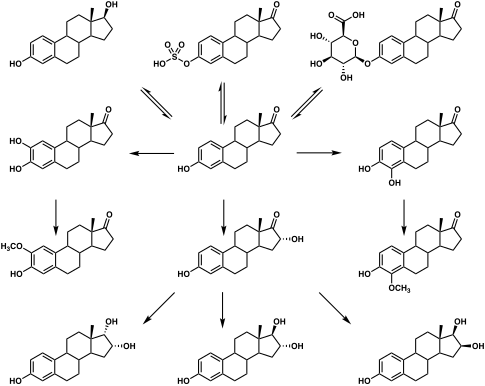
E1S itself is biologically inactive, with less than 1% of the relative binding affinity of estradiol for the ERα and ERβ,[4] but it can be converted by steroid sulfatase (also known as estrogen sulfatase) into estrone, an estrogen.[5] Simultaneously, estrogen sulfotransferases, including SULT1A1 and SULT1E1, convert estrone to E1S, resulting in an equilibrium between the two steroids in various tissues.[1][5] Estrone can also be transformed by 17β-hydroxysteroid dehydrogenases into the more potent estrogen estradiol.[1] E1S levels are much higher than those of estrone and estradiol, and it is thought to serve as a long-lasting reservoir for estrone and estradiol in the body.[1][6][7]
E1S has been found to transactivate the estrogen receptor at physiologically relevant concentrations.[8][9] This was diminished with co-application of irosustat (STX-64), a steroid sulfatase inhibitor, indicating that transformation of estrone sulfate into estrone is involved.[8][9]
Unlike unconjugated estradiol and estrone, which are lipophilic compounds, E1S is more hydrophilic, and in relation to this, it is unable to diffuse through the lipid bilayer of cell membranes.[10][11] Instead, estrone sulfate is transported into cells by active transport via organic-anion-transporting polypeptides (OATPs), including OATP1A2, OATP1B1, OATP1B3, OATP1C1, OATP2B1, and OATP3A1.[10][11]
E1S may be involved in the pathophysiology of breast cancer, benign breast disease, endometrial cancer, ovarian cancer, and prostate cancer.[1]
Chemistry
E1S, also known as estrone 3-sulfate or as estra-1,3,5(10)-trien-17-one 3-sulfate, is a naturally occurring estrane steroid and a derivative of estrone.[12] It is an estrogen conjugate or ester, and is specifically the C3 sulfate ester of estrone.[12] Related estrogen conjugates include estradiol sulfate, estriol sulfate, estrone glucuronide, estradiol glucuronide, and estriol glucuronide, while related steroid conjugates include dehydroepiandrosterone sulfate and pregnenolone sulfate.
Biochemistry
Metabolism of estrone sulfate in humans
|
Levels
Circulating levels of estrone sulfate range from a low of 0.13 ng/mL in postmenopausal women to a high of 105 ng/mL in pregnant women during the third trimester.[8]
References
- ^ a b c d e Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Crit Rev Clin Lab Sci. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.
- ^ Lobo, Rogerio A. (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 768–. ISBN 978-0-08-055309-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ^ a b Falcone, Tommaso; Hurd, William W. (22 May 2013). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 5–6. ISBN 978-1-4614-6837-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Melmed, Shlomo; Polonsky, Kenneth S.; Larsen, P. Reed; Kronenberg, Henry M. (11 November 2015). Williams Textbook of Endocrinology (13th ed.). Elsevier Health Sciences. pp. 607–. ISBN 978-0-323-34157-8.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Greenblatt, James M.; Brogan, Kelly (27 April 2016). Integrative Therapies for Depression: Redefining Models for Assessment, Treatment and Prevention. CRC Press. pp. 198–. ISBN 978-1-4987-0230-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Bjerregaard-Olesen C, Ghisari M, Kjeldsen LS, Wielsøe M, Bonefeld-Jørgensen EC (January 2016). "Estrone sulfate and dehydroepiandrosterone sulfate: Transactivation of the estrogen and androgen receptor". Steroids. 105: 50–8. doi:10.1016/j.steroids.2015.11.009. PMID 26666359.
- ^ a b Clark, Barbara J.; Prough, Russell A.; Klinge, Carolyn M. (2018). "Mechanisms of Action of Dehydroepiandrosterone". 108: 29–73. doi:10.1016/bs.vh.2018.02.003. ISSN 0083-6729.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Africander D, Storbeck KH (May 2018). "Steroid metabolism in breast cancer: Where are we and what are we missing?". Mol. Cell. Endocrinol. 466: 86–97. doi:10.1016/j.mce.2017.05.016. PMID 28527781.
- ^ a b Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (October 2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr. Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 900–. ISBN 978-1-4757-2085-3.
Further reading
- Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Critical Reviews in Clinical Laboratory Sciences. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.