Inositol
| Inositol[1] | |
|---|---|
| |
| Chemical name | cis-1,2,3,5-trans-4,6-Cyclohexanehexol |
| Other names | myo-Inositol, Cyclohexanehexol, Cyclohexitol, Dambose, Inosital, Inosite, iso-Inositol, Inositene, Inositina, i-Inositol, Inositol, MI, Meat sugar, Mesoinosit, Mesoinosite, meso-Inositol, Mesol, Mesovit, Myoinosite, Mouse antialopecia factor, Nucite, Phaseomannite, Phaseomannitol Rat antispectacled eye factor, Scyllite |
| Chemical formula | C6H12O6 |
| Molecular mass | 180.16 g/mol |
| CAS number | [87-89-8] |
| Density | 1.752 g/cm3 |
| Melting point | 225-227 °C |
| SMILES | O[C@H]1[C@H](O)[C@@H](O) [C@H](O)[C@H](O)[C@@H]1O |
| Disclaimer and references | |
Inositol, or cis-1,2,3,5-trans-4,6-cyclohexanehexol, is a cyclic polyalcohol that plays an important role as a second messenger in a cell, in the form of inositol phosphates. It is found in many foods, particularly in cereals with high bran content.
It is classified as a member of the vitamin B complex, though it is not considered a vitamin itself because it can be synthesized by the human body.
Structure
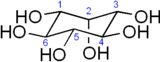
The chemical formula of inositol is Template:Carbon6Template:Hydrogen12Template:Oxygen6. In its most stable geometry, the inositol ring is in the chair conformation. There are nine stereoisomers, all of which may be referred to as inositol; however, the most common in nature has a structure in which the 1st, 3rd, 4th, 5th and 6th hydroxyls are positioned equatorially to the plane of the ring, while the 2nd hydroxyl group is positioned axially to the plane of the ring.
Synthesis
Inositol is synthesized from glucose-6-phosphate (G6P) in two steps. First, G6P is isomerized by INYNA1 to myo-inositol 1-phosphate, which is then dephosphorylated by IMPA1 to yield inositol.
Function
Inositol is involved in many biological processes, including:
- cytoskeleton assembly
- nerve guidance (Epsin)
- intracellular calcium (Ca2+) concentration control
- cell membrane potential maintenance
- serotonin activity modulation
- breakdown of fats and reducing blood cholesterol[citation needed]
- gene expression (Wu 2003 and OShea 2003, both in Science)
Clinical implications
Some preliminary results of studies on inositol supplements show promising results for people suffering from problems such as bulimia, panic disorder and bipolar depression.
Inositol has been found in double-blind studies to be an effective treatment for obsessive-compulsive disorder (OCD). It is equal in effectiveness to SSRIs and is virtually free from side effects.[2][3][4]
Illicit uses
Inositol powder can be used in small proportions as a cutting agent for cocaine HCL or methamphetamine (crystal meth). It has an almost identical appearance when in powder form and portrays similar qualities when heated. This, in addition to the fact that it adds almost no discernable taste or feel to either drug regardless the method of use, makes it an ideal cutting agent. Cutting either drug at any point in the distribution increases volume of the street product and increases dealer profits. However, at higher cut levels the inositol becomes somewhat noticeable in that the quality of the product is obviously diminished.
See also
- inositol monophosphate
- inositol triphosphate
- inositol pentakisphosphate
- inositol hexaphosphate
- inositol triphosphate receptor
References
- ^ Merck Index, 11th Edition, 4883.
- ^ Palatnik A, Frolov K, Fux M, Benjamin J (2001). "Double-blind, controlled, crossover trial of inositol versus fluvoxamine for the treatment of panic disorder". Journal of Clinical Psychopharmacology. 21 (3): 335–339.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fux M, Levine J, Aviv A, Belmaker RH (1996). "Inositol treatment of obsessive-compulsive disorder". American Journal of Psychiatry. 153 (9): 1219–21.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Di Paolo G, De Camilli P (2006). "Phosphoinositides in cell regulation and membrane dynamics". Nature. 443 (7112): 651–657.
