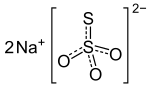
Sodium thiosulfate
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium thiosulfate
| |
| Other names
Sodium hyposulfite
Hyposulphite of soda | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.970 |
| E number | E539 (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na2S2O3 | |
| Molar mass | 158.11 g/mol (anhydrous) 248.18 g/mol (pentahydrate) |
| Appearance | White crystals |
| Odor | Odorless |
| Density | 1.667 g/cm3 |
| Melting point | 48.3 °C (118.9 °F; 321.4 K) (pentahydrate) |
| Boiling point | 100 °C (212 °F; 373 K) (pentahydrate, - 5H2O decomposition) |
| 70.1 g/100 mL (20 °C)[1] 231 g/100 mL (100 °C) | |
| Solubility | negligible in alcohol |
Refractive index (nD)
|
1.489 |
| Structure | |
| monoclinic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula Na2S2O3.xH2O. Typically it is available as the white or colorless pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. It is also often called sodium hyposulfite or hypo.[2]
Sodium thiosulfate is used in gold mining, water treatment, analytic chemistry, the development of silver-based photographic film and prints, and medicine. The medical uses of sodium thiosulfate include treatment of cyanide poisoning and pityriasis.[3] It is on the World Health Organization's List of Essential Medicines.[4]
Uses
Medical uses
The main medical use of sodium thiosulfate is the treatment of cyanide poisoning.[3] Other uses include topical treatment of ringworm and tinea versicolor,[3][5] and treating some side effects of hemodialysis[6] and chemotherapy.[7]
Iodometry
In analytical chemistry, the most important use comes because the thiosulfate anion reacts stoichiometrically with iodine in aqueous solution, reducing it to iodide as the thiosulfate is oxidized to tetrathionate:
- 2 S2O32− + I2 → S4O62− + 2 I−
Due to the quantitative nature of this reaction, as well as because Na2S2O3·5H2O has an excellent shelf-life, it is used as a titrant in iodometry. Na2S2O3·5H2O is also a component of iodine clock experiments.
This particular use can be set up to measure the oxygen content of water through a long series of reactions in the Winkler test for dissolved oxygen. It is also used in estimating volumetrically the concentrations of certain compounds in solution (hydrogen peroxide, for instance) and in estimating the chlorine content in commercial bleaching powder and water.
Photographic processing
Silver halides, e.g., AgBr, typical components of photographic emulsions, dissolve upon treatment with aqueous thiosulfate:
- 2 S2O32− + AgBr → [Ag(S2O3)2]3− + Br−
This application as a photographic fixer was discovered by John Herschel. It is used for both film and photographic paper processing; the sodium thiosulfate is known as a photographic fixer, and is often referred to as 'hypo', from the original chemical name, hyposulphite of soda.[8] Ammonium thiosulfate is typically preferred to sodium thiosulfate for this application.[2]
Gold extraction
Sodium thiosulfate and ammonium thiosulfate are a component of an alternative lixiviants to cyanide for extraction of gold.[9][2] Thiosulfate forms strong soluble complexes with gold(I) ions, [Au(S2O3)2]3−. The advantages of this approach are that (i) thiosulfate is essentially nontoxic and (ii) that ore types that are refractory to gold cyanidation (e.g. carbonaceous or Carlin-type ores) can be leached by thiosulfate. Some problems with this alternative process include the high consumption of thiosulfate, and the lack of a suitable recovery technique, since [Au(S2O3)2]3− does not adsorb to activated carbon, which is the standard technique used in gold cyanidation to separate the gold complex from the ore slurry.
Neutralizing chlorinated water
It is used to dechlorinate tap water including lowering chlorine levels for use in aquariums, swimming pools, and spas (e.g., following superchlorination) and within water treatment plants to treat settled backwash water prior to release into rivers.[2] The reduction reaction is analogous to the iodine reduction reaction.
In pH testing of bleach substances, sodium thiosulfate neutralizes the color-removing effects of bleach and allows one to test the pH of bleach solutions with liquid indicators. The relevant reaction is akin to the iodine reaction: thiosulfate reduces the hypochlorite (active ingredient in bleach) and in so doing becomes oxidized to sulfate. The complete reaction is:
- 4 NaClO + Na2S2O3 + 2 NaOH → 4 NaCl + 2 Na2SO4 + H2O
Similarly, sodium thiosulfate reacts with bromine, removing the free bromine from solution. Solutions of sodium thiosulfate are commonly used as a precaution in chemistry laboratories when working with bromine and for the safe disposal of bromine, iodine, or other strong oxidizers.
Structure
Two polymorphs are known of the pentahydrate. The anhydrous salt exists in several polymorphs.[2] In the solid state, the thiosulfate anion is tetrahedral in shape and is notionally derived by replacing one of the oxygen atoms by a sulfur atom in a sulfate anion. The S-S distance indicates a single bond, implying that the terminal sulfur holds a significant negative charge and the S-O interactions have more double-bond character.
Production
On an industrial scale, sodium thiosulfate is produced chiefly from liquid waste products of sodium sulfide or sulfur dye manufacture.[10]
In the laboratory, this salt can be prepared by heating an aqueous solution of sodium sulfite with sulfur or by boiling aqueous sodium hydroxide and sulfur according to this equation:[11]
- 6 NaOH + 4 S → 2 Na2S + Na2S2O3 + 3 H2O
Principal reactions
Upon heating to 300 °C, it decomposes to sodium sulfate and sodium polysulfide:
- 4 Na2S2O3 → 3 Na2SO4 + Na2S5
Thiosulfate salts characteristically decompose upon treatment with acids. Initial protonation occurs at sulfur. When the protonation is conducted in diethyl ether at −78 °C, H2S2O3 (thiosulfuric acid) can be obtained. It is a somewhat strong acid with pKas of 0.6 and 1.7 for the first and second dissociations, respectively.
Under normal conditions, acidification of solutions of this salt excess with even dilute acids results in complete decomposition to sulfur, sulfur dioxide, and water:[10]
- Na2S2O3 + 2 HCl → 2 NaCl + S + SO2 + H2O
This reaction is known as a "clock reaction", because when the sulfur reaches a certain concentration, the solution turns from colourless to a pale yellow. This reaction has been employed to generate colloidal sulfur. This process is used to demonstrate the concept of reaction rate in chemistry classes.
Aluminium cation reaction
Sodium thiosulfate is also used in analytical chemistry.[citation needed] It can, when heated with a sample containing aluminium cations, produce a white precipitate:
- 2 Al3+ + 3 S2O32− + 3 H2O → 3 SO2 + 3 S + 2 Al(OH)3
Organic chemistry
Alkylation of sodium thiosulfate gives S-alkylthiosulfates, which are called Bunte salts.[12] The alkylthiosulfates are susceptible to hydrolysis, affording the thiol. This reaction is illustrated by one synthesis of thioglycolic acid:
- ClCH2CO2H + Na2S2O3 → Na[O3S2CH2CO2H] + NaCl
- Na[O3S2CH2CO2H] + H2O → HSCH2CO2H + NaHSO4
References
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c d e "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2012. doi:10.1002/14356007.a25_477. ISBN 978-3527306732.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ a b c WHO Model Formulary 2008 (PDF). World Health Organization. 2009. p. 66. ISBN 9789241547659. Retrieved 8 January 2017.
- ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- ^ Peter J. Sunenshine, Robert A. Schwartz, and Camila K. Janniger (2002): "Tinea versicolor". International Journal of Dermatology, volume 37, issue 9, pages 648-655. doi:10.1046/j.1365-4362.1998.00441.x
- ^ {M. Auriemma, A. Carbone, L. Di Liberato, A. Cupaiolo, C. Caponio, C. De Simone, A. Tulli, M. Bonomini, and P. Amerio (2011): "Treatment of cutaneous calciphylaxis with sodium thiosulfate: two case reports and a review of the literature". American Journal of Clinical Dermatology, volume 12, issue 5, pages=339–346. PMID 21834598
- ^ (2005): "Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels". Journal of Pharmacology and Experimental Therapeutics, volume 314, issue=3, pages 1052–1058. PMID 15951398 doi:10.1124/jpet.105.087601
- ^ Charles Robert Gibson (1908). The Romance of Modern Photography, Its Discovery & Its Achievements. Seeley & Co. p. 37.
- ^ Aylmore MG, Muir DM (2001). "Thiosulfate Leaching of Gold - a Review". Minerals Engineering. 14 (2): 135–174. doi:10.1016/s0892-6875(00)00172-2.
- ^ a b Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5
- ^ Gordin, H. M. (1913). Elementary Chemistry, Volume I. Inorganic Chemistry. Chicago: Medico-Dental Publishing Co. pp. 162 & 287–288.
- ^ "Sulfide Synthesis in Preparation of Unsymmetrical Dialkyl Disulfides: Sec-butyl Isopropyl Disulfide". Org. Synth. 58: 147. 1978. doi:10.15227/orgsyn.058.0147.
{{cite journal}}: Unknown parameter|authors=ignored (help)

