Marine life


Marine life, or sea life or ocean life, is the plants, animals and other organisms that live in the salt water of the sea or ocean, or the brackish water of coastal estuaries. At a fundamental level, marine life affects the nature of the planet. Marine organisms produce oxygen. Shorelines are in part shaped and protected by marine life, and some marine organisms even help create new land.
Most life forms evolved initially in marine habitats. By volume, oceans provide about 90 percent of the living space on the planet.[1] The earliest vertebrates were believed to have appeared in the form of fish, which live exclusively in water. [2]Some of these evolved into amphibians which spend portions of their lives in water and portions on land. Other fish evolved into land mammals and subsequently returned to the ocean as cetaceans, pinnipedss, and sirenians. Plant forms such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton, and particularly phytoplankton, are key primary producers forming the general foundation of the ocean food chain.
Marine invertebrates exhibit a wide range of modifications to survive in poorly oxygenated waters, including breathing tubes (see insect and mollusc siphons) and gills. Fish have gills instead of lungs, although some species of fish, such as the lungfish, have both. Marine mammals, such as dolphins, whales, otters, and seals need to surface periodically to breathe air. Some amphibians are able to absorb oxygen through their skin.
A total of 230,000 documented marine species exist, including about 20,000 species of marine fish, with some two million marine species yet to be documented.[3] Marine species range in size from the microscopic, including plankton and phytoplankton which can be as small as 0.02 micrometres, to huge cetaceans (whales, dolphins and porpoises), including the blue whale – the largest known animal reaching up to 33 metres (108 ft) in length.[4][5] Marine microorganisms, including bacteria and viruses, constitute about 70% of the total marine biomass.
Water

There is no life without water – the "solvent of life".[6] The Nobel Prize winner Albert Szent-Györgyi referred to water as the mater und matrix: the mother and womb of life.[7]
The abundance of surface water on Earth is a unique feature in the Solar System. Earth's hydrosphere consists chiefly of the oceans, but technically includes all water surfaces in the world, including inland seas, lakes, rivers, and underground waters down to a depth of 2,000 metres (6,600 ft) The deepest underwater location is Challenger Deep of the Mariana Trench in the Pacific Ocean, having a depth of 10,911 metres (6.780 mi).[note 1][8]
The mass of the oceans is approximately 1.35×1018 metric tons, or about 1/4400 of Earth's total mass. The oceans cover an area of 3.618×108 km2 with a mean depth of 3682 m, resulting in an estimated volume of 1.332×109 km3.[9] If all of Earth's crustal surface was at the same elevation as a smooth sphere, the depth of the resulting world ocean would be about 2.7 kilometres (1.7 mi).[10][11]

About 97.5% of the water on Earth is saline; the remaining 2.5% is fresh water. Most fresh water – about 68.7% – is present as ice in ice caps and glaciers.[12] The average salinity of Earth's oceans is about 35 grams (1.2 oz) of salt per kilogram of seawater (3.5% salt).[13] Most of the salt in the ocean comes from the weathering and erosion of rocks on land.[14] Some salts are released from volcanic activity or extracted from cool igneous rocks.[15] The oceans are also a reservoir of dissolved atmospheric gases, which are essential for the survival of many aquatic life forms.[16] Sea water has an important influence on the world's climate, with the oceans acting as a large heat reservoir.[17] Shifts in the oceanic temperature distribution can cause significant weather shifts, such as the El Niño-Southern Oscillation.[18]
Altogether the ocean occupies 71 percent of the world surface,[1] averaging nearly 3.7 kilometres (2.3 mi) in depth.[19] By volume, the ocean provides about 90 percent of the living space on the planet.[1] The science fiction writer Arthur C. Clarke has pointed out it would be more appropriate to refer to planet Earth as planet Ocean.[20][21]
Evolution
−4500 — – — – −4000 — – — – −3500 — – — – −3000 — – — – −2500 — – — – −2000 — – — – −1500 — – — – −1000 — – — – −500 — – — – 0 — |
| |||||||||||||||||||||||||||||||||||||||||||||
The Earth is about 4.54 billion years old.[22][23][24] The earliest undisputed evidence of life on Earth dates from at least 3.5 billion years ago,[25][26] during the Eoarchean Era after a geological crust started to solidify following the earlier molten Hadean Eon. Microbial mat fossils have been found in 3.48 billion-year-old sandstone in Western Australia.[27][28][29] Other early physical evidence of a biogenic substance is graphite in 3.7 billion-year-old metasedimentary rocks discovered in Western Greenland[30] as well as "remains of biotic life" found in 4.1 billion-year-old rocks in Western Australia.[31][32] According to one of the researchers, "If life arose relatively quickly on Earth … then it could be common in the universe."[31]
All organisms on Earth are descended from a common ancestor or ancestral gene pool.[33][34] Highly energetic chemistry is thought to have produced a self-replicating molecule around 4 billion years ago, and half a billion years later the last common ancestor of all life existed.[35] The current scientific consensus is that the complex biochemistry that makes up life came from simpler chemical reactions.[36] The beginning of life may have included self-replicating molecules such as RNA[37] and the assembly of simple cells.[38] In 2016 scientists reported a set of 355 genes from the last universal common ancestor (LUCA) of all life, including microorganisms, living on Earth.[39]
Current species are a stage in the process of evolution, with their diversity the product of a long series of speciation and extinction events.[40] The common descent of organisms was first deduced from four simple facts about organisms: First, they have geographic distributions that cannot be explained by local adaptation. Second, the diversity of life is not a set of completely unique organisms, but organisms that share morphological similarities. Third, vestigial traits with no clear purpose resemble functional ancestral traits and finally, that organisms can be classified using these similarities into a hierarchy of nested groups—similar to a family tree.[41] However, modern research has suggested that, due to horizontal gene transfer, this "tree of life" may be more complicated than a simple branching tree since some genes have spread independently between distantly related species.[42][43]
Past species have also left records of their evolutionary history. Fossils, along with the comparative anatomy of present-day organisms, constitute the morphological, or anatomical, record.[44] By comparing the anatomies of both modern and extinct species, paleontologists can infer the lineages of those species. However, this approach is most successful for organisms that had hard body parts, such as shells, bones or teeth. Further, as prokaryotes such as bacteria and archaea share a limited set of common morphologies, their fossils do not provide information on their ancestry.

More recently, evidence for common descent has come from the study of biochemical similarities between organisms. For example, all living cells use the same basic set of nucleotides and amino acids.[46] The development of molecular genetics has revealed the record of evolution left in organisms' genomes: dating when species diverged through the molecular clock produced by mutations.[47] For example, these DNA sequence comparisons have revealed that humans and chimpanzees share 98% of their genomes and analysing the few areas where they differ helps shed light on when the common ancestor of these species existed.[48]
Prokaryotes inhabited the Earth from approximately 3–4 billion years ago.[49][50] No obvious changes in morphology or cellular organisation occurred in these organisms over the next few billion years.[51] The eukaryotic cells emerged between 1.6–2.7 billion years ago. The next major change in cell structure came when bacteria were engulfed by eukaryotic cells, in a cooperative association called endosymbiosis.[52][53] The engulfed bacteria and the host cell then underwent coevolution, with the bacteria evolving into either mitochondria or hydrogenosomes.[54] Another engulfment of cyanobacterial-like organisms led to the formation of chloroplasts in algae and plants.[55]

The history of life was that of the unicellular eukaryotes, prokaryotes and archaea until about 610 million years ago when multicellular organisms began to appear in the oceans in the Ediacaran period.[49][56] The evolution of multicellularity occurred in multiple independent events, in organisms as diverse as sponges, brown algae, cyanobacteria, slime moulds and myxobacteria.[57] In January 2016, scientists reported that, about 800 million years ago, a minor genetic change in a single molecule called GK-PID may have allowed organisms to go from a single cell organism to one of many cells.[58]
Soon after the emergence of these first multicellular organisms, a remarkable amount of biological diversity appeared over approximately 10 million years, in an event called the Cambrian explosion. Here, the majority of types of modern animals appeared in the fossil record, as well as unique lineages that subsequently became extinct.[59] Various triggers for the Cambrian explosion have been proposed, including the accumulation of oxygen in the atmosphere from photosynthesis.[60]
About 500 million years ago, plants and fungi started colonising the land. Evidence for the appearance of the first land plants occurs in the Ordovician, around 450 million years ago, in the form of fossil spores.[61] Land plants began to diversify in the Late Silurian, from around 430 million years ago.[62] The colonisation of the land by plants was soon followed by arthropods and other animals.[63] Insects were particularly successful and even today make up the majority of animal species.[64] Amphibians first appeared around 364 million years ago, followed by early amniotes and birds around 155 million years ago (both from "reptile"-like lineages), mammals around 129 million years ago, homininae around 10 million years ago and modern humans around 250,000 years ago.[65][66][67] However, despite the evolution of these large animals, smaller organisms similar to the types that evolved early in this process continue to be highly successful and dominate the Earth, with the majority of both biomass and species being prokaryotes.[68]
Estimates on the number of Earth's current species range from 10 million to 14 million,[69] of which about 1.2 million have been documented and over 86 percent have not yet been described.[70]
Marine microorganisms
Microorganisms make up about 70% of the marine biomass.[71] A microorganism, or microbe, is a microscopic organism too small to be recognised with the naked eye. It can be single-celled[72] or multicellular. Microorganisms are diverse and include all bacteria and archaea, most protozoa such as algae, fungi and certain microscopic animals such as rotifers.
Many macroscopic animals and plants have microscopic juvenile stages. Some microbiologists also classify viruses (and viroids) as microorganisms, but others consider these as nonliving.[73][74]
Microorganisms are crucial to nutrient recycling in ecosystems as they act as decomposers. Some microorganisms are pathogenic, causing disease and even death in plants and animals.[75] As inhabitants of the largest environment on Earth, microbial marine systems drive changes in every global system. Microbes are responsible for virtually all the photosynthesis that occurs in the ocean, as well as the cycling of carbon, nitrogen, phosphorus and other nutrients and trace elements.[76]


Microscopic life undersea is diverse and still poorly understood, such as for the role of viruses in marine ecosystems.[77] Most marine viruses are bacteriophages, which are harmless to plants and animals, and are essential to the regulation of saltwater and freshwater ecosystems.[78] They infect and destroy bacteria in aquatic microbial communities, and are the most important mechanism of recycling carbon in the marine environment. The organic molecules released from the dead bacterial cells stimulate fresh bacterial and algal growth.[79] Viral activity may also contribute to the biological pump, the process whereby carbon is sequestered in the deep ocean.[80]

A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[81] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[82][83]
Microscopic organisms live throughout the biosphere. The mass of prokaryote microorganisms — which includes bacteria and archaea, but not the nucleated eukaryote microorganisms — may be as much as 0.8 trillion tons of carbon (of the total biosphere mass, estimated at between 1 and 4 trillion tons).[84] Barophilic marine microbes have been found at more than a depth of 10,000 m (33,000 ft; 6.2 mi) in the Mariana Trench, the deepest spot in the Earth's oceans.[85] Single-celled life forms have been found in the deepest part of the Mariana Trench, by the Challenger Deep, at depths of 11,034 m (36,201 ft; 6.856 mi).[86][87][88] Microorganisms live inside rocks up to 580 m (1,900 ft; 0.36 mi) below the sea floor under 2,590 m (8,500 ft; 1.61 mi) of ocean off the coast of the northwestern United States,[87][89] as well as 2,400 m (7,900 ft; 1.5 mi) beneath the seabed off Japan.[90] The greatest known temperature at which microbial life can exist is 122 °C (252 °F) (Methanopyrus kandleri).[91] In 2014, scientists confirmed the existence of microorganisms living 800 m (2,600 ft; 0.50 mi) below the ice of Antarctica.[92][93] According to one researcher, "You can find microbes everywhere — they're extremely adaptable to conditions, and survive wherever they are."[87]
Marine viruses
Viruses are small infectious agents that do not have their own metabolism and can replicate only inside the living cells of other organisms.[94] Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea.[95] The linear size of the average virus is about one one-hundredth that of the average bacterium. Most viruses cannot be seen with an optical microscope so electron microscopes are used instead.[96]
Viruses are found wherever there is life and have probably existed since living cells first evolved.[97] The origin of viruses is unclear because they do not form fossils, so molecular techniques have been used to compare the DNA or RNA of viruses and are a useful means of investigating how they arose.[98]
Viruses are now recognised as ancient and as having origins that pre-date the divergence of life into the three domains.[99] But the origins of viruses in the evolutionary history of life are unclear: some may have evolved from plasmids—pieces of DNA that can move between cells—while others may have evolved from bacteria. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity.[100]
Opinions differ on whether viruses are a form of life or organic structures that interact with living organisms.[101] They are considered by some to be a life form, because they carry genetic material, reproduce by creating multiple copies of themselves through self-assembly, and evolve through natural selection. However they lack key characteristics such as a cellular structure generally considered necessary to count as life. Because they possess some but not all such qualities, viruses have been described as replicators[102] and as "organisms at the edge of life".[103]
Bacteriophages, often just called phages, are viruses that are parasitic on bacteria. Marine phages are obligate parasitic agents in marine bacteria such as cyanobacteria.[104] They are a common and diverse group of viruses and are the most abundant biological entity in marine environments, because their hosts, bacteria, are typically the numerically dominant cellular life in the sea. Generally there are about 1 million to 10 million viruses in each mL of seawater, or about ten times more double-stranded DNA viruses than there are cellular organisms,[105][106] although estimates of viral abundance in seawater can vary over a wide range.[107][108] Tailed bacteriophages appear to dominate marine ecosystems in number and diversity of organisms,[104] and bacteriophages belonging to the families Corticoviridae,[109] Inoviridae[110] and Microviridae[111] are also known to infect diverse marine bacteria.

Microorganisms make up about 70% of the marine biomass.[71] It is estimated viruses kill 20% of this biomass each day and that there are 15 times as many viruses in the oceans as there are bacteria and archaea. Viruses are the main agents responsible for the rapid destruction of harmful algal blooms,[112] which often kill other marine life.[113] The number of viruses in the oceans decreases further offshore and deeper into the water, where there are fewer host organisms.[80]
There are also archaean viruses which replicate within archaea: these are double-stranded DNA viruses with unusual and sometimes unique shapes.[114][115] These viruses have been studied in most detail in the thermophilic archaea, particularly the orders Sulfolobales and Thermoproteales.[116]
Viruses are an important natural means of transferring genes between different species, which increases genetic diversity and drives evolution.[100] It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the last universal common ancestor of life on Earth.[117] Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.[80]
Marine bacteria

Bacteria constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a number of shapes, ranging from spheres to rods and spirals. Bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste,[118] and the deep portions of Earth's crust. Bacteria also live in symbiotic and parasitic relationships with plants and animals.
Once regarded as plants constituting the class Schizomycetes, bacteria are now classified as prokaryotes. Unlike cells of animals and other eukaryotes, bacterial cells do not contain a nucleus and rarely harbour membrane-bound organelles. Although the term bacteria traditionally included all prokaryotes, the scientific classification changed after the discovery in the 1990s that prokaryotes consist of two very different groups of organisms that evolved from an ancient common ancestor. These evolutionary domains are called Bacteria and Archaea.[119]
The ancestors of modern bacteria were unicellular microorganisms that were the first forms of life to appear on Earth, about 4 billion years ago. For about 3 billion years, most organisms were microscopic, and bacteria and archaea were the dominant forms of life.[120][121] Although bacterial fossils exist, such as stromatolites, their lack of distinctive morphology prevents them from being used to examine the history of bacterial evolution, or to date the time of origin of a particular bacterial species. However, gene sequences can be used to reconstruct the bacterial phylogeny, and these studies indicate that bacteria diverged first from the archaeal/eukaryotic lineage.[122] Bacteria were also involved in the second great evolutionary divergence, that of the archaea and eukaryotes. Here, eukaryotes resulted from the entering of ancient bacteria into endosymbiotic associations with the ancestors of eukaryotic cells, which were themselves possibly related to the Archaea.[53][123] This involved the engulfment by proto-eukaryotic cells of alphaproteobacterial symbionts to form either mitochondria or hydrogenosomes, which are still found in all known Eukarya. Later on, some eukaryotes that already contained mitochondria also engulfed cyanobacterial-like organisms. This led to the formation of chloroplasts in algae and plants. There are also some algae that originated from even later endosymbiotic events. Here, eukaryotes engulfed a eukaryotic algae that developed into a "second-generation" plastid.[124][125] This is known as secondary endosymbiosis.
-
The marine Thiomargarita namibiensis, largest known bacterium
-
The chloroplasts of glaucophytes have a peptidoglycan layer, evidence suggesting their endosymbiotic origin from cyanobacteria.[126]
-
Bacteria can be beneficial. This Pompeii worm, an extremophile found only at hydrothermal vents, has a protective cover of bacteria.
The largest known bacterium, the marine Thiomargarita namibiensis, can be visible to the naked eye and sometimes attains 0.75 mm (750 μm).[127][128]
Marine archaea

The archaea (Greek for ancient[130]) constitute a domain and kingdom of single-celled microorganisms. These microbes are prokaryotes, meaning they have no cell nucleus or any other membrane-bound organelles in their cells.
Archaea were initially classified as bacteria, but this classification is outdated.[131] Archaeal cells have unique properties separating them from the other two domains of life, Bacteria and Eukaryota. The Archaea are further divided into multiple recognized phyla. Classification is difficult because the majority have not been isolated in the laboratory and have only been detected by analysis of their nucleic acids in samples from their environment.
Archaea and bacteria are generally similar in size and shape, although a few archaea have very strange shapes, such as the flat and square-shaped cells of Haloquadratum walsbyi.[132] Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably the enzymes involved in transcription and translation. Other aspects of archaeal biochemistry are unique, such as their reliance on ether lipids in their cell membranes, such as archaeols. Archaea use more energy sources than eukaryotes: these range from organic compounds, such as sugars, to ammonia, metal ions or even hydrogen gas. Salt-tolerant archaea (the Haloarchaea) use sunlight as an energy source, and other species of archaea fix carbon; however, unlike plants and cyanobacteria, no known species of archaea does both. Archaea reproduce asexually by binary fission, fragmentation, or budding; unlike bacteria and eukaryotes, no known species forms spores.
Archaea are particularly numerous in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet. Archaea are a major part of Earth's life and may play roles in both the carbon cycle and the nitrogen cycle.
-
Halobacteria, found in water near saturated with salt, are now recognised as archaea.
-
Flat, square-shaped cells of the archaea Haloquadratum walsbyi
-
Methanosarcina barkeri, a marine archaea that produces methane
-
Thermophiles, such as Pyrolobus fumarii, survive well over 100°C
-
Drawing of another marine thermophile, Pyrococcus furiosus
Marine protists
All living organisms can be grouped as either prokaryotes or eukaryotes. Life originated as single-celled prokaryotes and later evolved into the more complex eukaryotes. In contrast to prokaryotic cells, eukaryotic cells are highly organised. Prokaryotes are the bacteria and archaea, while eukaryotes are the other life forms — plants, animals, fungi and protists. Plants, animals or fungi are usually multi-celled. Protists are eukaryotes that cannot be classified as plants, animals or fungi. They are usually single-celled and microscopic.
Protists include amoebae, ciliates, red algae, euglena, phytoplankton such as diatoms and dinoflagellates, and slime molds. Several algae species are multicellular protists, and some marine slime molds have unique life cycles that involve switching between unicellular, colonial, and multicellular forms.[133] Protists are a highly diverse group of organisms currently organised into 18 phyla, but they are not easy to classify.[134][135] Studies have shown a high protist diversity exists in oceans, deep sea-vents and river sediments, which suggests a large number of eukaryotic microbial communities have yet to be discovered.[136][137]
-
Marine diatoms
-
Single-celled alga, Gephyrocapsa oceanica
-
Red algae, Cyanidium sp.
-
Silicified cell wall of a diatom Amphora sp.
-
Dino-
flagellate
Marine microanimals
As juveniles, animals develop from microscopic stages, which can include spores, eggs and larvae. At least one microscopic animal group, the parasitic cnidarian Myxozoa, is unicellular in its adult form, and includes marine species. Other adult marine microanimals are multicellular. Microscopic adult arthropods are more commonly found inland in freshwater, but there are marine species as well. Microscopic adult marine crustaceans include some copepods, cladocera and water bears. Some marine nematodes and rotifers are also too small to be seen with the naked eye, as are many loricifera, including the recently discovered anaerobic species that spend their lives in an anoxic environment.[138][139] Copepods contribute more to the secondary productivity and carbon sink of the world oceans than any other group of organisms.
- Marine micro-animals
-
Over 10,000 marine species are copepods, small, often microscopic crustaceans
-
Darkfield photo of a gastrotrich, which form a phylum of worm-like animals living mostly between particles of sediment
-
Armoured Pliciloricus enigmaticus of the phylum Loricifera live in the spaces between marine gravel
Marine algae and plants

Microscopic algae and plants provide important habitats for life, sometimes acting as hiding and foraging places for larval forms of larger fish and invertebrates. Algal life is widespread and very diverse under the ocean. Microscopic photosynthetic algae contribute a larger proportion of the world's photosynthetic output than all the terrestrial forests combined. Most of the niche occupied by sub plants on land is actually occupied by macroscopic algae in the ocean, such as Sargassum and kelp, which are commonly known as seaweeds that create kelp forests.
Plants that survive in the sea are often found in shallow waters, such as the seagrasses (examples of which are eelgrass, Zostera, and turtle grass, Thalassia). These plants have adapted to the high salinity of the ocean environment. The intertidal zone is also a good place to find plant life in the sea, where mangroves or cordgrass or beach grass might grow.
-
Volvox is a microscopic green freshwater alga with spherical symmetry. Young colonies can be seen inside the larger ones.
Marine fungi


Over 1500 species of fungi are known from marine environments.[140] These are parasitic on marine algae or animals, or are saprobes on algae, corals, protozoan cysts, sea grasses, wood and other substrata, and can also be found in sea foam.[141] Spores of many species have special appendages which facilitate attachment to the substratum.[142] A very diverse range of unusual secondary metabolites is produced by marine fungi.[143]
Mycoplankton are saprotropic members of the plankton communities of marine and freshwater ecosystems.[144][145] They are composed of filamentous free-living fungi and yeasts that are associated with planktonic particles or phytoplankton.[146] Similar to bacterioplankton, these aquatic fungi play a significant role in heterotrophic mineralization and nutrient cycling.[147] Mycoplankton can be up to 20 mm in diameter and over 50 mm in length.[148]
In a typical milliliter of seawater, there are approximately 103 to 104 fungal cells.[149] This number is greater in coastal ecosystems and estuaries due to nutritional runoff from terrestrial communities. The greatest diversity and number of species of mycoplankton is found in surface waters (< 1000 m), and the vertical profile depends on the abundance of phytoplankton.[150][151] Furthermore, this difference in distribution may vary between seasons due to nutrient availability.[152] Marine fungi survive in a constant oxygen deficient environment, and therefore depend on oxygen diffusion by turbulence and oxygen generated by photosynthetic organisms.[153]
Marine fungi can be classified as:[153]
- Lower fungi - adapted to marine habitats (zoosporic fungi, including mastigomycetes: oomycetes and chytridiomycetes)
- Higher fungi - filamentous, modified to planktonic lifestyle (hyphomycetes, ascomycetes, basidiomycetes)
Most mycoplankton species are higher fungi.[150]
Lichens are mutualistic associations between a fungus, usually an ascomycete, and an alga or a cyanobacterium. Several lichens are found in marine environments.[154] Many more occur in the splash zone, where they occupy different vertical zones depending on how tolerant they are to submersion.[155] Fossil marine lichens 600 million years old have been discovered in the late Neoproterozoic marine phosphate rocks in the sedimentary, fossil-rich Doushantuo Formation in China.[156]
According to fossil records, fungi date back to the late Proterozoic era 900-570 million years ago. It has been hypothesized that mycoplankton evolved from terrestrial fungi, likely in the Paleozoic era (390 million years ago).[157]
Invertebrates

The earliest animals were marine invertebrates, that is, vertebrates came later. Animals are multicellular eukaryotes,[note 2] and are distinguished from plants, algae, and fungi by lacking cell walls.[158] Marine invertebrates are animals that inhabit a marine environment apart from the vertebrate members of the chordate phylum; invertebrates lack a vertebral column. Some have evolved a shell or a hard exoskeleton.
The earliest animal fossils may belong to the genus Dickinsonia,[159] 571 million to 541 million years ago.[160] Individual Dickinsonia typically resemble a bilaterally symmetrical ribbed oval. They kept growing until they were covered with sediment or otherwise killed,[161] and spent most of their lives with their bodies firmly anchored to the sediment.[162] Their taxonomic affinities are presently unknown, but their mode of growth is consistent with a bilaterian affinity.[163]
Apart from Dickinsonia, the earliest widely accepted animal fossils are the rather modern-looking cnidarians (the group that includes jellyfish, sea anemones and Hydra), possibly from around 580 Ma[164] The Ediacara biota, which flourished for the last 40 million years before the start of the Cambrian,[165] were the first animals more than a very few centimetres long. Like Dickinsonia, many were flat with a "quilted" appearance, and seemed so strange that there was a proposal to classify them as a separate kingdom, Vendozoa.[166] Others, however, have been interpreted as early molluscs (Kimberella[167][168]), echinoderms (Arkarua[169]), and arthropods (Spriggina,[170] Parvancorina[171]). There is still debate about the classification of these specimens, mainly because the diagnostic features which allow taxonomists to classify more recent organisms, such as similarities to living organisms, are generally absent in the Ediacarans. However, there seems little doubt that Kimberella was at least a triploblastic bilaterian animal, in other words, an animal significantly more complex than the cnidarians.[172]
Small shelly fauna are a very mixed collection of fossils found between the Late Ediacaran and Middle Cambrian periods. The earliest, Cloudina, shows signs of successful defense against predation and may indicate the start of an evolutionary arms race. Some tiny Early Cambrian shells almost certainly belonged to molluscs, while the owners of some "armor plates," Halkieria and Microdictyon, were eventually identified when more complete specimens were found in Cambrian lagerstätten that preserved soft-bodied animals.[173]
Phyla


Invertebrates are grouped into different phyla. Informally phyla can be thought of as a way of grouping organisms according to their body plan.[175][176]: 33 A body plan refers to a blueprint which describes the shape or morphology of an organism, such as its symmetry, segmentation and the disposition of its appendages. The idea of body plans originated with vertebrates, which were grouped into one phylum. But the vertebrate body plan is only one of many, and invertebrates consist of many phyla or body plans. The history of the discovery of body plans can be seen as a movement from a worldview centred on vertebrates, to seeing the vertebrates as one body plan among many. Among the pioneering zoologists, Linnaeus identified two body plans outside the vertebrates; Cuvier identified three; and Haeckel had four, as well as the Protista with eight more, for a total of twelve. For comparison, the number of phyla recognised by modern zoologists has risen to 35.[176]
Historically body plans were thought of as having evolved rapidly during the Cambrian explosion,[177] but a more nuanced understanding of animal evolution suggests a gradual development of body plans throughout the early Palaeozoic and beyond.[178] More generally a phylum can be defined in two ways: as described above, as a group of organisms with a certain degree of morphological or developmental similarity (the phenetic definition), or a group of organisms with a certain degree of evolutionary relatedness (the phylogenetic definition).[178]
In the 1970s there was already a debate about whether the emergence of the modern phyla was "explosive" or gradual but hidden by the shortage of Precambrian animal fossils.[173] A re-analysis of fossils from the Burgess Shale lagerstätte increased interest in the issue when it revealed animals, such as Opabinia, which did not fit into any known phylum. At the time these were interpreted as evidence that the modern phyla had evolved very rapidly in the Cambrian explosion and that the Burgess Shale's "weird wonders" showed that the Early Cambrian was a uniquely experimental period of animal evolution.[179] Later discoveries of similar animals and the development of new theoretical approaches led to the conclusion that many of the "weird wonders" were evolutionary "aunts" or "cousins" of modern groups[180]—for example that Opabinia was a member of the lobopods, a group which includes the ancestors of the arthropods, and that it may have been closely related to the modern tardigrades.[181] Nevertheless, there is still much debate about whether the Cambrian explosion was really explosive and, if so, how and why it happened and why it appears unique in the history of animals.[182]
Arthropods total about 1,113,000 described extant species, molluscs about 85,000 and chordates about 52,000.[183][184]
Marine sponges

Sponges are animals of the phylum Porifera (Modern Latin for bearing pores[185]). They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells. They have unspecialized cells that can transform into other types and that often migrate between the main cell layers and the mesohyl in the process. Sponges do not have nervous, digestive or circulatory systems. Instead, most rely on maintaining a constant water flow through their bodies to obtain food and oxygen and to remove wastes.
Sponges are similar to other animals in that they are multicellular, heterotrophic, lack cell walls and produce sperm cells. Unlike other animals, they lack true tissues and organs, and have no body symmetry. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where it deposits nutrients, and leaves through a hole called the osculum. Many sponges have internal skeletons of spongin and/or spicules of calcium carbonate or silicon dioxide. All sponges are sessile aquatic animals. Although there are freshwater species, the great majority are marine (salt water) species, ranging from tidal zones to depths exceeding 8,800 m (5.5 mi).
While most of the approximately 5,000–10,000 known species feed on bacteria and other food particles in the water, some host photosynthesizing micro-organisms as endosymbionts and these alliances often produce more food and oxygen than they consume. A few species of sponge that live in food-poor environments have become carnivores that prey mainly on small crustaceans.[186]
-
Sponge biodiversity. There are four sponge species in this photo.
-
Venus' flower basket at a depth of 2572 meters
Linnaeus mistakenly identified sponges as plants in the order Algae.[187] For a long time thereafter sponges were assigned to a separate subkingdom, Parazoa (meaning beside the animals).[188] They are now classified as a paraphyletic phylum from which the higher animals have evolved.[189]
Marine cnidarians

Cnidarians (Greek for nettle) are distinguished by the presence of stinging cells, specialized cells that they use mainly for capturing prey. Cnidarians include corals, sea anemones, jellyfish and hydrozoans. They form a phylum containing over 10,000[190] species of animals found exclusively in aquatic (mainly marine) environments. Their bodies consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion and respiration.
Fossil cnidarians have been found in rocks formed about 580 million years ago. Fossils of cnidarians that do not build mineralized structures are rare. Scientists currently think cnidarians, ctenophores and bilaterians are more closely related to calcareous sponges than these are to other sponges, and that anthozoans are the evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Cnidarians are the simplest animals in which the cells are organised into tissues.[191] The starlet sea anemone is used as a model organism in research.[192] It is easy to care for in the laboratory and a protocol has been developed which can yield large numbers of embryos on a daily basis.[193] There is a remarkable degree of similarity in the gene sequence conservation and complexity between the sea anemone and vertebrates.[193] In particular, genes concerned in the formation of the head in vertebrates are also present in the anemone.[194][195]
-
Sea anemones are common in tidepools
-
Their tentacles sting and paralyse small fish
-
If an island sinks below the sea, coral growth can keep pace with the rising water and form an atoll
-
Turritopsis dohrnii achieves biological immortality by transfering its cells back to childhood [198][199]
Marine worms

Worms (Old English for serpent) form a number of phylums. Their body plan typically involves long cylindrical tube-like bodies and no limbs. Marine worms vary in size from microscopic to over 1 metre (3.3 ft) in length for some marine polychaete worms (bristle worms)[200] and up to 58 metres (190 ft) for the marine nemertean worm (bootlace worm).[201] Some marine worms occupy a small variety of parasitic niches, living inside the bodies of other animals, while others live more freely in the marine environment or by burrowing underground.
Different groups of marine worms are related only distantly, so they are found in several different phyla such as the Annelida (segmented worms), Chaetognatha (arrow worms), Hemichordata, and Phoronida (horseshoe worms). Many of these worms have specialized tentacles used for exchanging oxygen and carbon dioxide and also may be used for reproduction. Some marine worms are tube worms, such as the giant tube worm which lives in waters near underwater volcanoes and can withstand temperatures up to 90 degrees Celsius.
Platyhelminthes (flatworms) form another worm phylum which includes a class Cestoda of parasitic tapeworms. The marine tapeworm Polygonoporus giganticus, found in the gut of sperm whales, can grow to over 30 m (100 ft).[202][203]
Nematodes (roundworms) constitute a further worm phylum with tubular digestive systems and an opening at both ends.[204][205] Over 25,000 nematode species have been described,[206][207] of which more than half are parasitic. It has been estimated another million remain undescribed.[208] They are ubiquitous in marine, freshwater and terrestrial environments, where they often outnumber other animals in both individual and species counts. They are found in every part of the earth's lithosphere, from the top of mountains to the bottom of oceanic trenches.[209] By count they represent 90% of all animals on the ocean floor.[210] Their numerical dominance, often exceeding a million individuals per square meter and accounting for about 80% of all individual animals on earth, their diversity of life cycles, and their presence at various trophic levels point at an important role in many ecosystems.[211]
-
Giant tube worms cluster around hydrothermal vents
-
Nematodes are ubiquitous pseudocoelomates which can parasite marine plants and animals.
-
Bloodworms are typically found on the bottom of shallow marine waters
Echinoderms
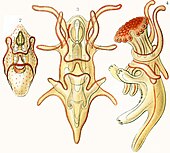
Echinoderms (Greek for spiny skin) is a phylum which contains only marine invertebrates. The adults are recognizable by their radial symmetry (usually five-point) and include starfish, sea urchins, sand dollars, and sea cucumbers, as well as the sea lilies.[212] Echinoderms are found at every ocean depth, from the intertidal zone to the abyssal zone. The phylum contains about 7000 living species,[213] making it the second-largest grouping of deuterostomes (a superphylum), after the chordates (which include the vertebrates, such as birds, fishes, mammals, and reptiles).
Echinoderms are unique among animals in having bilateral symmetry at the larval stage, but fivefold symmetry (pentamerism, a special type of radial symmetry) as adults.[214]
The echinoderms are important both biologically and geologically. Biologically, there are few other groupings so abundant in the biotic desert of the deep sea, as well as shallower oceans. Most echinoderms are able to regenerate tissue, organs, limbs, and reproduce asexually; in some cases, they can undergo complete regeneration from a single limb. Geologically, the value of echinoderms is in their ossified skeletons, which are major contributors to many limestone formations, and can provide valuable clues as to the geological environment. They were the most used species in regenerative research in the 19th and 20th centuries. Further, it is held by some scientists that the radiation of echinoderms was responsible for the Mesozoic Marine Revolution.
-
Echinoderm literally means "spiny skin", as this water melon sea urchin illustrates
-
The ochre sea star was the first keystone predator to be studied. They limit mussels which can overwhelm intertidal communities.[215]
-
Colorful sea lilies in shallow waters
-
Sea cucumbers filter feed on plankton and suspended solids
-
The benthopelagic sea cucumber can lift off the seafloor and journey as much as 1,000 m (3,300 ft) up the water column
-
This deep water sea cucumber, a sea pig, is the only echinoderm that uses legged locomotion.
Aside from the hard-to-classify Arkarua (a Precambrian animal with echinoderm-like pentamerous radial symmetry), the first definitive members of the phylum appeared near the start of the Cambrian.
Marine molluscs

Molluscs (Latin for soft) form a phylum with about 85,000 extant recognized species.[216] They are the largest marine phylum in terms of species count, comprising about 23% of all the named marine organisms.[217] Molluscs have more varied forms than other invertebrate phylums. They are highly diverse, not just in size and in anatomical structure, but also in behaviour and in habitat. The majority of species still live in the oceans, from the seashores to the abyssal zone, but some form a significant part of the freshwater fauna and the terrestrial ecosystems.
The mollusc phylum is divided into 9 or 10 taxonomic classes, two of which are extinct. These classes include gastropods, bivalves and cephalopods, as well as other lesser-known but distinctive classes. Gastropods with protective shells are referred to as snails, whereas gastropods without protective shells are referred to as slugs. Gastropods are by far the most numerous molluscs in terms of classified species, accounting for 80% of the total.[183] Bivalves include clams, oysters, cockles, mussels, scallops, and numerous other families. There are about 8,000 marine bivalves species (including brackish water and estuarine species), and about 1,200 freshwater species. Cephalopod include octopus, squid and cuttlefish. They are found in all oceans, and neurologically are the most advanced of the invertebrates.[218] About 800 living species of marine cephalopods have been identified,[219] and an estimated 11,000 extinct taxa have been described.[220] There are no fully freshwater cephalopods.[221]
-
Colossal squid, largest of all invertebrates[222]
-
The nautilus is a living fossil little changed since it evolved 500 million years ago as one of the first cephalopods.[223][224][225]
-
The sea snail Syrinx aruanus has the largest shell of any living gastropod
-
Molluscs usually have eyes. Bordering the edge of the mantle of a scallop, a bivalve mollusc, can be over 100 simple eyes.
-
Common mussel, another bivalve

Molluscs have such diverse shapes that many textbooks base their descriptions of molluscan anatomy on a generalized or hypothetical ancestral mollusc. This generalized mollusc is unsegmented and bilaterally symmetrical with an underside consisting of a single muscular foot.[226][227]: 484–628 Beyond that it has three further key features. Firstly, it has a muscular cloak called a mantle covering its viscera and containing a significant cavity used for breathing and excretion. A shell secreted by the mantle covers the upper surface.[227] Secondly (apart from bivalves) it has a rasping tongue called a radula used for feeding. Thirdly, it has a nervous system including a complex digestive system using microscopic, muscle-powered hairs called cilia to exude mucus. The generalized mollusc has two paired nerve cords (three in bivalves). The brain, in species that have one, encircles the esophagus. Most molluscs have eyes and all have sensors detecting chemicals, vibrations, and touch. The simplest type of molluscan reproductive system relies on external fertilization, but more complex variations occur. All produce eggs, from which may emerge trochophore larvae, more complex veliger larvae, or miniature adults. The depiction is rather similar to modern monoplacophorans, and some suggest it may resemble very early molluscs.[226]: 284–291 [226]: 298–300 [228][229]
Good evidence exists for the appearance of marine gastropods, cephalopods and bivalves in the Cambrian period 538.8 to 485.4 million years ago. However, the evolutionary history both of molluscs' emergence from the ancestral Lophotrochozoa and of their diversification into the well-known living and fossil forms are still subjects of vigorous debate among scientists.
Marine arthropods
Arthropods (Greek for jointed feet) have an exoskeleton (external skeleton), a segmented body, and jointed appendages (paired appendages). They form a phylum which includes insects, arachnids, myriapods, and crustaceans. Arthropods are characterized by their jointed limbs and cuticle made of chitin, often mineralised with calcium carbonate. The arthropod body plan consists of segments, each with a pair of appendages. The rigid cuticle inhibits growth, so arthropods replace it periodically by moulting. Their versatility has enabled them to become the most species-rich members of all ecological guilds in most environments.
Extant marine arthropods range in size from the microscopic crustacean Stygotantulus to the Japanese spider crab. Arthropods' primary internal cavity is a hemocoel, which accommodates their internal organs, and through which their haemolymph - analogue of blood - circulates; they have open circulatory systems. Like their exteriors, the internal organs of arthropods are generally built of repeated segments. Their nervous system is "ladder-like", with paired ventral nerve cords running through all segments and forming paired ganglia in each segment. Their heads are formed by fusion of varying numbers of segments, and their brains are formed by fusion of the ganglia of these segments and encircle the esophagus. The respiratory and excretory systems of arthropods vary, depending as much on their environment as on the subphylum to which they belong.
Arthropod vision relies on various combinations of compound eyes and pigment-pit ocelli: in most species the ocelli can only detect the direction from which light is coming, and the compound eyes are the main source of information. Arthropods also have a wide range of chemical and mechanical sensors, mostly based on modifications of the many setae (bristles) that project through their cuticles. Arthropod methods of reproduction are diverse: terrestrial species use some form of internal fertilization while marine species lay eggs using either internal or external fertilization. Arthropod hatchlings vary from miniature adults to grubs that lack jointed limbs and eventually undergo a total metamorphosis to produce the adult form.
-
Fossil trilobite. Trilobites first appeared about 521 Ma. They were highly successful and were found everywhere in the ocean for 270 Ma.[231]
-
The Anomalocaris ("abnormal shrimp") was one of the first apex predators and first appeared about 515 Ma
-
Horseshoe crabs are living fossils, essentially unchanged for 450 Ma
-
The largest known arthropod, the sea scorpion Jaekelopterus rhenaniae, has been found in estuarine strata from about 390 Ma. It was up to 2.5 m (8.2 ft) long.[232][233]
- Crustaceans
-
Many crustaceans are very small, like this tiny amphipod, and make up a significant part of the ocean's zooplankton
-
Mantis shrimp have the most advanced eyes in the animal kingdom,[234] and smash prey by swinging their club-like raptorial claws.[235]
-
The Tasmanian giant crab is long-lived and slow-growing, making it vulnerable to overfishing.[236]
-
The Japanese spider crab has the longest leg span of any arthropod, reaching 5.5 metres (18 ft) from claw to claw.[237]
The evolutionary ancestry of arthropods dates back to the Cambrian period. The group is generally regarded as monophyletic, and many analyses support the placement of arthropods with cycloneuralians (or their constituent clades) in a superphylum Ecdysozoa. Overall however, the basal relationships of Metazoa are not yet well resolved. Likewise, the relationships between various arthropod groups are still actively debated.
Others
-
Lobopodia are an extinct group of worm-like taxa with stubby legs that resemble modern velvet worms
-
Tardigrades are a phylum of eight-legged, segmented micro-animals able to survive in extreme conditions
- Non-craniate (non-vertebrate) chordates: Cephalochordate, Tunicata and Haikouella. These invertebrates are close relatives of the vertebrates.
- Non-craniate chordates are close relatives of vertebrates
-
The lancelet, a small translucent fish-like Cephalochordate, is the closest living invertebrate relative of the vertebrates.[238][239]
-
Fluorescent-colored sea squirts, Rhopalaea crassa. Tunicates may provide clues to vertebrate (and therefore human) ancestry.[240]
-
Pyrosomes are free-floating bioluminescent tunicates made up of hundreds of individuals
-
Salp chain
-
Gill slits in an acorn worm (left) and tunicate (right)
Vertebrates
Fish
Fish anatomy includes a two-chambered heart, operculum, swim bladder, scales, eyes adapted to seeing underwater, and secretory cells that produce mucous. Fish breathe by extracting oxygen from water through gills. Fins propel and stabilize the fish in the water. Fish fall into two main groups: fish with bony skeletons and fish with cartilaginous skeletons. Over 33,000 species of fish have been described as of 2015,[241] and about 20,000 are marine.[242]
Jawless fish
Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of jawless fish.[243] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[244] only 18 species of lampreys are actually parasitic.[245] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[246] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[247]
-
Lampreys are often parasitic and have a toothed, funnel-like sucking mouth
-
The extinct Pteraspidomorphi, ancestral to jawed vertebrates
Pteraspidomorphi is an extinct class of early jawless fish ancestral to jawed vertebrates. The few characteristics they share with the latter are now considered as primitive for all vertebrates.
Cartilaginous
Cartilaginous fish, such as sharks and rays, have jaws and skeletons made of cartilage rather than bone. Megalodon is an extinct species of shark that lived about 28 to 1.5 Ma. It looked much like a stocky version of the great white shark, but was much larger with fossil lengths reaching 20.3 metres (67 ft).[248] Found in all oceans[249] it was one of the largest and most powerful predators in vertebrate history,[248] and probably had a profound impact on marine life.[250] The Greenland shark has the longest known lifespan of all vertebrates, about 400 years.[251]
-
Cartilaginous fishes may have evolved from spiny sharks
-
The extinct megalodon resembled a giant great white shark
-
The Greenland shark lives longer than any other vertebrate
-
The whale shark
-
Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[252]
-
The giant manta ray, largest ray in the world, has been targeted by fisheries and is now vulnerable.[253]
Bony
Bony fish have jaws and skeletons made of bone rather than cartilage. Bony fish also have a hard, bony plate covering their gills called an operculum, and can have distinct rays, or spines, in their fins. About 90% of the world's fish species are bony fish.
-
The extinct Leedsichthys is the largest known bony fish
Marine tetrapods
Amphibians
Amphibians mostly require fresh water to reproduce. A few inhabit brackish water, but there are no true marine amphibians.[254] There have been reports, however, of amphibians invading marine waters, such as a Black Sea invasion by the natural hybrid Pelophylax esculentus reported in 2010.[255]
Reptiles
Reptiles (Late Latin for creeping or crawling) form a class Reptilia of tetrapods, vertebrates that have either four limbs or have four-limbed ancestors. Some reptiles are more closely related to birds than other reptiles, and many scientists prefer to make Reptilia a monophyletic group which includes the birds.[256][257][258][259] Extant non-avian reptiles which inhabit or frequent the sea include sea turtles, sea snakes, terrapins, the marine iguana, and the saltwater crocodile. Currently, of the approximately 12,000 extant reptile species and sub-species, only about 100 of are classed as marine reptiles.[260]
Unlike amphibians, reptiles do not have an aquatic larval stage. Most reptiles are oviparous, although several species of squamates are viviparous, as were some extinct aquatic clades[261] — the fetus develops within the mother, contained in a placenta rather than an eggshell. As amniotes, reptile eggs are surrounded by membranes for protection and transport, which adapt them to reproduction on dry land. Many of the viviparous species feed their fetuses through various forms of placenta analogous to those of mammals, with some providing initial care for their hatchlings.
Except for some sea snakes, most extant marine reptiles are oviparous and need to return to land to lay their eggs. Apart from sea turtles, the species usually spend most of their lives on or near land rather than in the ocean. Sea snakes generally prefer shallow waters nearby land, around islands, especially waters that are somewhat sheltered, as well as near estuaries.[262][263] Unlike land snakes, sea snakes have evolved flattened tails which help them swim.[264]
-
Marine snakes have flattened tails
-
The ancient Ichthyosaurus communis independently evolved flippers similar to dolphins
Some extinct marine reptiles, such as ichthyosaurs, evolved to be viviparous and had no requirement to return to land. Ichthyosaurs resembled dolphins. They first appeared about 245 million years ago and disappeared about 90 million years ago. The terrestrial ancestor of the ichthyosaur had no features already on its back or tail that might have helped along the evolutionary process. Yet the ichthyosaur developed a dorsal and tail fin which improved its ability to swim.[265] The biologist Stephen Jay Gould said the ichthyosaur was his favourite example of convergent evolution.[266] The earliest marine reptiles arose in the Permian. During the Mesozoic many groups of reptiles became adapted to life in the seas, including ichthyosaurs, plesiosaurs, mosasaurs, nothosaurs, placodonts, sea turtles, thalattosaurs and thalattosuchians. Marine reptiles were less numerous after mass extinction at the end of the Cretaceous.
Birds
Marine birds are adapted to life within the marine environment. They are often called seabirds. While marine birds vary greatly in lifestyle, behaviour and physiology, they often exhibit striking convergent evolution, as the same environmental problems and feeding niches have resulted in similar adaptations. Examples include albatross, penguins, gannets, and auks.
In general, marine birds live longer, breed later and have fewer young than terrestrial birds do, but they invest a great deal of time in their young. Most species nest in colonies, which can vary in size from a few dozen birds to millions. Many species are famous for undertaking long annual migrations, crossing the equator or circumnavigating the Earth in some cases. They feed both at the ocean's surface and below it, and even feed on each other. Marine birds can be highly pelagic, coastal, or in some cases spend a part of the year away from the sea entirely. Some marine birds plummet from heights, plunging through the water leaving vapour-like trails, similar to that of fighter planes.[267] Gannets plunge into the water at up to 100 kilometres per hour (60 mph). They have air sacs under their skin in their face and chest which act like bubble-wrap, cushioning the impact with the water.
-
European herring gull attack herring schools from above
-
Gentoo penguin swimming underwater
-
Gannets "divebomb" at high speed
-
Albatrosses range over huge areas of ocean and regularly circle the globe.
The first marine birds evolved in the Cretaceous period, and modern marine bird families emerged in the Paleogene.
Mammals

There are about 130 living and recently extinct marine mammal species such as seals, dolphins, whales, manatees, sea otters and polar bears.[268] They do not represent a distinct taxon or systematic grouping, but are instead unified by their reliance on the marine environment for feeding. Both cetaceans and sirenians are fully aquatic and therefore are obligate water dwellers. Seals and sea-lions are semiaquatic; they spend the majority of their time in the water, but need to return to land for important activities such as mating, breeding and molting. In contrast, both otters and the polar bear are much less adapted to aquatic living. Their diet varies considerably as well; some may eat zooplankton, others may eat fish, squid, shellfish, sea-grass and a few may eat other mammals.
In a process of convergent evolution, marine mammals such as dolphins and whales redeveloped their body plan to parallel the streamlined fusiform body plan of pelagic fish. Front legs became flippers and back legs disappeared, a dorsal fin reappeared and the tail morphed into a powerful horizontal fluke. This body plan is an adaptation to being an active predator in a high drag environment. A parallel convergence occurred with the now extinct ichthyosaur.[269]
-
Endangered blue whale, largest animal ever[270]
-
Killer whales jumping
Plankton

Plankton are a diverse group of organisms that live in the water column of large bodies of water and that cannot swim against a current.[272] They provide a crucial source of food to many large aquatic organisms, such as fish and whales.
These organisms include drifting or floating bacteria, archaea, algae, protozoa and animals that inhabit, for example, the pelagic zone of oceans, seas, or bodies of fresh water. Essentially, plankton are defined by their ecological niche rather than any phylogenetic or taxonomic classification.
Though many planktonic species are microscopic in size, plankton includes organisms covering a wide range of sizes, including large organisms such as jellyfish.[273]
The role of phytoplankton is better understood due to their critical position as the most numerous primary producers on Earth. Phytoplankton are categorized into cyanobacteria (also called blue-green algae/bacteria), various types of algae (red, green, brown, and yellow-green), diatoms, dinoflagellates, euglenoids, coccolithophorids, cryptomonads, chrysophytes, chlorophytes, prasinophytes, and silicoflagellates.
- Phytoplankton
-
Phytoplankton are the foundation of the oceanic food chain.
-
They come in many shapes and sizes.
-
Diatoms are one of the most common types of phytoplankton
-
Green cyanobacteria scum washed up on a rock in California
-
Algal bloom off south England.
Zooplankton tend to be somewhat larger, and not all are microscopic. Many Protozoa are zooplankton, including dinoflagellates, zooflagellates, foraminiferans, and radiolarians. Some of these (such as dinoflagellates) are also phytoplankton; the distinction between plants and animals often breaks down in very small organisms. Other zooplankton include cnidarians, ctenophores, chaetognaths, molluscs, arthropods, urochordates, and annelids such as polychaetes.
- Zooplankton
-
Marine amphipod
Many larger animals begin their life as zooplankton before they become large enough to take their adult forms.
- Spawn, larvae and juveniles
-
Male star coral releasing sperm into the water
-
Ocean sunfish larva
-
Juvenile planktonic squid
Land interactions

Food webs

-
Whale pump nutrient cycle
-
Waterbird food web
Biogeochemical cycles
-
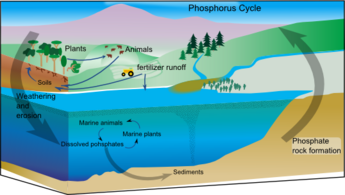
Marine phosphorus cycle

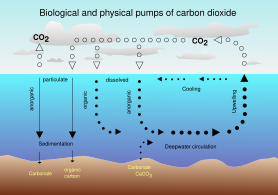
In 2000 a team of microbiologists led by Edward DeLong made a crucial discovery in the understanding of the marine carbon and energy cycles. They discovered a gene in several species of bacteria[277][278] responsible for production of the protein rhodopsin, previously unheard of in the domain Bacteria. These proteins found in the cell membranes are capable of converting light energy to biochemical energy due to a change in configuration of the rhodopsin molecule as sunlight strikes it, causing the pumping of a proton from inside out and a subsequent inflow that generates the energy.[279]
Biodiversity and extinction events

Biodiversity is the result of over three billion years of evolution. Until approximately 600 million years ago, all life consisted of archaea, bacteria, protozoans and similar single-celled organisms. The history of biodiversity during the Phanerozoic (the last 540 million years), starts with rapid growth during the Cambrian explosion – a period during which nearly every phylum of multicellular organisms first appeared. Over the next 400 million years or so, invertebrate diversity showed little overall trend and vertebrate diversity shows an overall exponential trend.[281]
However, more than 99 percent of all species that ever lived on Earth, amounting to over five billion species,[282] are estimated to be extinct.[283][284] These extinctions occur at an uneven rate. The dramatic rise in diversity has been marked by periodic, massive losses of diversity classified as mass extinction events.[281] Mass extinction events occur when life undergoes precipitous global declines. Most diversity and biomass on earth is found among the microorganisms, which are difficult to measure. Recorded extinction events are therefore based on the more easily observed changes in the diversity and abundance of larger multicellular organisms, rather than the total diversity and abundance of life.[285] Marine fossils are mostly used to measure extinction rates because of their superior fossil record and stratigraphic range compared to land organisms.
Based on the fossil record, the background rate of extinctions on Earth is about two to five taxonomic families of marine animals every million years. The Great Oxygenation Event was perhaps the first major extinction event. Since the Cambrian explosion five further major mass extinctions have significantly exceeded the background extinction rate.[286] The worst was the Permian-Triassic extinction event, 251 million years ago. Vertebrates took 30 million years to recover from this event.[287] In addition to these major mass extinctions there are numerous minor ones, as well as the current ongoing mass-extinction caused by human activity, the Holocene extinction sometimes called the "sixth extinction".
Marine biology

During the sixth century BC, the Greek philosopher Xenophanes (570-475 BC) recognised that some fossil shells were remains of shellfish. He used this to argue that what was at the time dry land was once under the sea.[288] This was an important step in advancing from simply stating an idea to backing it with evidence and observation.[289]
Later, during the fourth century BC, another Greek philosopher Aristotle (384–322 BC) attempted a comprehensive classification of animals which included systematic descriptions of many marine species,[290][291] and particularly species found in the Mediterranean Sea.[292] These pioneering works include History of Animals, a general biology of animals, Parts of Animals, a comparative anatomy and physiology of animals, and Generation of Animals, on developmental biology. The most striking passages are about the sea-life visible from observation on Lesbos and available from the catches of fishermen. His observations on catfish, electric fish (Torpedo) and angler-fish are detailed, as is his writing on cephalopods, namely, Octopus, Sepia (cuttlefish) and the paper nautilus (Argonauta argo). His description of the hectocotyl arm, used in sexual reproduction, was widely disbelieved until its rediscovery in the 19th century. He separated aquatic mammals from fish, and knew that sharks and rays were part of a group he called Selachē (selachians).[293] He gave accurate descriptions of the ovoviviparous embryological development of the hound shark Mustelus mustelus.[294] His classification of living things contains elements which were still in use in the 19th century. What the modern zoologist would call vertebrates and invertebrates, Aristotle called "animals with blood" and "animals without blood" (he did not know that complex invertebrates do make use of hemoglobin, but of a different kind from vertebrates). He divided animals with blood into live-bearing (mammals), and egg-bearing (birds and fish). Invertebrates ("animals without blood") he divided into insects, crustacea (further divided into non-shelled – cephalopods – and shelled) and testacea (molluscs).[295][296]
In contemporary times, marine life is a field of study both in marine biology and in biological oceanography. In biology many phyla, families and genera have some species that live in the sea and others that live on land. Marine biology classifies species based on the environment rather than on taxonomy. For this reason marine biology encompasses not only organisms that live only in a marine environment, but also other organisms whose lives revolve around the sea. Biological oceanography is the study of how organisms affect and are affected by the physics, chemistry, and geology of the oceanographic system. Biological oceanography mostly focuses on the microorganisms within the ocean; looking at how they are affected by their environment and how that affects larger marine creatures and their ecosystem.[297] Biological oceanography is similar to marine biology, but it studies ocean life from a different perspective. Biological oceanography takes a bottom up approach in terms of the food web, while marine biology studies the ocean from a top down perspective. Biological oceanography mainly focuses on the ecosystem of the ocean with an emphasis on plankton: their diversity (morphology, nutritional sources, motility, and metabolism); their productivity and how that plays a role in the global carbon cycle; and their distribution (predation and life cycle).[297][298][299] Biological oceanography also investigates the role of microbes in food webs, and how humans impact the ecosystems in the oceans.[297]
See also
 Marine life portal
Marine life portal- Blue Planet - David Attenborough
- Census of Marine Life
- Effects of global warming on marine mammals
- Hydrobiology
- List of marine aquarium invertebrate species
- Milky seas effect
- Natural Geography in Shore Areas (NaGISA)
- Ocean Biogeographic Information System (OBIS)
Notes
- ^ This is the measurement taken by the vessel Kaikō in March 1995 and is considered the most accurate measurement to date. See the Challenger Deep article for more details.
- ^ Myxozoa were thought to be an exception, but are now thought to be heavily modified members of the Cnidaria. Jímenez-Guri, Eva; Philippe, Hervé; Okamura, Beth; Holland, Peter W. H. (6 July 2007). "Buddenbrockia Is a Cnidarian Worm". Science. 317 (5834): 116–118. Bibcode:2007Sci...317..116J. doi:10.1126/science.1142024. ISSN 0036-8075. PMID 17615357. Retrieved 3 September 2008.
{{cite journal}}: Invalid|ref=harv(help)
References
- ^ a b c "National Oceanic and Atmospheric Administration – Ocean". NOAA. Retrieved 20 February 2019.
- ^ https://www.livescience.com/46262-fossil-ancestor-jawed-vertebrates.html
- ^ Drogin, Bob (2 August 2009). "Mapping an ocean of species". Los Angeles Times. Retrieved 18 August 2009.
- ^ Paul, Gregory S. (2010). "The Evolution of Dinosaurs and their World". The Princeton Field Guide to Dinosaurs. Princeton: Princeton University Press. p. 19.
- ^ Bortolotti, Dan (2008). Wild Blue: A Natural History of the World's Largest Animal. St. Martin's Press.
- ^ Agre, P (2006). "The aquaporin water channels". Proceedings of the American Thoracic Society. 3 (1): 5–13. doi:10.1513/pats.200510-109JH. PMC 2658677. PMID 16493146.
- ^ Collins J. C. (1991) The Matrix of Life: A View of Natural Molecules from the Perspective of Environmental Water Molecular Presentations. ISBN 9780962971907.
- ^ "7,000 m Class Remotely Operated Vehicle KAIKO 7000". Japan Agency for Marine-Earth Science and Technology (JAMSTEC). Retrieved 7 June 2008.
- ^ Charette, Matthew A.; Smith, Walter H. F. (June 2010). "The Volume of Earth's Ocean" (PDF). Oceanography. 23 (2): 112–14. doi:10.5670/oceanog.2010.51. Archived from the original (PDF) on 2 August 2013. Retrieved 6 June 2013.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "sphere depth of the ocean – hydrology". Encyclopædia Britannica. Retrieved 12 April 2015.
- ^ "Third rock from the Sun – restless Earth". NASA's Cosmos. Retrieved 12 April 2015.
- ^ Perlman, Howard (17 March 2014). "The World's Water". USGS Water-Science School. Retrieved 12 April 2015.
- ^ Kennish, Michael J. (2001). Practical handbook of marine science. Marine science series (3rd ed.). CRC Press. p. 35. ISBN 978-0-8493-2391-1.
- ^ "Why is the ocean salty?".
- ^ Mullen, Leslie (11 June 2002). "Salt of the Early Earth". NASA Astrobiology Magazine. Archived from the original on 22 July 2007. Retrieved 14 March 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Morris, Ron M. "Oceanic Processes". NASA Astrobiology Magazine. Archived from the original on 15 April 2009. Retrieved 14 March 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Scott, Michon (24 April 2006). "Earth's Big heat Bucket". NASA Earth Observatory. Retrieved 14 March 2007.
- ^ Sample, Sharron (21 June 2005). "Sea Surface Temperature". NASA. Archived from the original on 3 April 2013. Retrieved 21 April 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Volumes of the World's Oceans from ETOPO1". NOAA. Archived from the original on 11 March 2015. Retrieved 20 February 2019.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Planet "Earth": We Should Have Called It "Sea" Quote Invertigator, 25 January 2017.
- ^ Unveiling Planet Ocean NASA Science, 14 March 2002.
- ^ "Age of the Earth". United States Geological Survey. 9 July 2007. Retrieved 31 May 2015.
- ^ Dalrymple 2001, pp. 205–221
- ^ Manhesa, Gérard; Allègre, Claude J.; Dupréa, Bernard; Hamelin, Bruno (May 1980). "Lead isotope study of basic-ultrabasic layered complexes: Speculations about the age of the earth and primitive mantle characteristics". Earth and Planetary Science Letters. 47 (3): 370–382. Bibcode:1980E&PSL..47..370M. doi:10.1016/0012-821X(80)90024-2. ISSN 0012-821X.
- ^ Schopf, J. William; Kudryavtsev, Anatoliy B.; Czaja, Andrew D.; Tripathi, Abhishek B. (5 October 2007). "Evidence of Archean life: Stromatolites and microfossils". Precambrian Research. 158 (3–4): 141–155. Bibcode:2007PreR..158..141S. doi:10.1016/j.precamres.2007.04.009. ISSN 0301-9268.
- ^ Raven & Johnson 2002, p. 68
- ^ Borenstein, Seth (13 November 2013). "Oldest fossil found: Meet your microbial mom". Excite. Yonkers, N.Y.: Mindspark Interactive Network. Associated Press. Retrieved 31 May 2015.
- ^ Pearlman, Jonathan (13 November 2013). "'Oldest signs of life on Earth found'". The Daily Telegraph. London: Telegraph Media Group. Retrieved 15 December 2014.
- ^ Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (16 November 2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Billion-Year-Old Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12): 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. ISSN 1531-1074. PMC 3870916. PMID 24205812.
- ^ Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25–28. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025. ISSN 1752-0894.
- ^ a b Borenstein, Seth (19 October 2015). "Hints of life on what was thought to be desolate early Earth". Associated Press. Retrieved 9 October 2018.
- ^ Bell, Elizabeth A.; Boehnike, Patrick; Harrison, T. Mark; et al. (24 November 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 112 (47): 14518–14521. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. ISSN 0027-8424. PMC 4664351. PMID 26483481. Retrieved 30 December 2015.
- ^ Penny, David; Poole, Anthony (December 1999). "The nature of the last universal common ancestor". Current Opinion in Genetics & Development. 9 (6): 672–677. doi:10.1016/S0959-437X(99)00020-9. ISSN 0959-437X. PMID 10607605.
- ^ Theobald, Douglas L. (13 May 2010). "A formal test of the theory of universal common ancestry". Nature. 465 (7295): 219–222. Bibcode:2010Natur.465..219T. doi:10.1038/nature09014. ISSN 0028-0836. PMID 20463738.
- ^ Doolittle, W. Ford (February 2000). "Uprooting the Tree of Life" (PDF). Scientific American. 282 (2): 90–95. Bibcode:2000SciAm.282b..90D. doi:10.1038/scientificamerican0200-90. ISSN 0036-8733. PMID 10710791. Archived from the original (PDF) on 7 September 2006. Retrieved 5 April 2015.
- ^ Peretó, Juli (March 2005). "Controversies on the origin of life" (PDF). International Microbiology. 8 (1): 23–31. ISSN 1139-6709. PMID 15906258. Archived from the original (PDF) on 24 August 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Joyce, Gerald F. (11 July 2002). "The antiquity of RNA-based evolution". Nature. 418 (6894): 214–221. Bibcode:2002Natur.418..214J. doi:10.1038/418214a. ISSN 0028-0836. PMID 12110897.
- ^ Trevors, Jack T.; Psenner, Roland (December 2001). "From self-assembly of life to present-day bacteria: a possible role for nanocells". FEMS Microbiology Reviews. 25 (5): 573–582. doi:10.1111/j.1574-6976.2001.tb00592.x. ISSN 1574-6976. PMID 11742692.
- ^ Wade, Nicholas (25 July 2016). "Meet Luca, the Ancestor of All Living Things". New York Times. Retrieved 25 July 2016.
- ^ Bapteste, Eric; Walsh, David A. (June 2005). "Does the 'Ring of Life' ring true?". Trends in Microbiology. 13 (6): 256–261. doi:10.1016/j.tim.2005.03.012. ISSN 0966-842X. PMID 15936656.
- ^ Darwin 1859, p. 1
- ^ Doolittle, W. Ford; Bapteste, Eric (13 February 2007). "Pattern pluralism and the Tree of Life hypothesis". Proceedings of the National Academy of Sciences of the United States of America. 104 (7): 2043–2049. Bibcode:2007PNAS..104.2043D. doi:10.1073/pnas.0610699104. ISSN 0027-8424. PMC 1892968. PMID 17261804.
- ^ Kunin, Victor; Goldovsky, Leon; Darzentas, Nikos; Ouzounis, Christos A. (July 2005). "The net of life: Reconstructing the microbial phylogenetic network". Genome Research. 15 (7): 954–959. doi:10.1101/gr.3666505. ISSN 1088-9051. PMC 1172039. PMID 15965028.
- ^ Jablonski, David (25 June 1999). "The Future of the Fossil Record". Science. 284 (5423): 2114–2116. doi:10.1126/science.284.5423.2114. ISSN 0036-8075. PMID 10381868.
- ^ Ciccarelli, Francesca D.; Doerks, Tobias; von Mering, Christian; et al. (3 March 2006). "Toward Automatic Reconstruction of a Highly Resolved Tree of Life". Science. 311 (5765): 1283–1287. Bibcode:2006Sci...311.1283C. CiteSeerX 10.1.1.381.9514. doi:10.1126/science.1123061. ISSN 0036-8075. PMID 16513982.
- ^ Mason, Stephen F. (6 September 1984). "Origins of biomolecular handedness". Nature. 311 (5981): 19–23. Bibcode:1984Natur.311...19M. doi:10.1038/311019a0. ISSN 0028-0836. PMID 6472461.
- ^ Wolf, Yuri I.; Rogozin, Igor B.; Grishin, Nick V.; Koonin, Eugene V. (1 September 2002). "Genome trees and the tree of life". Trends in Genetics. 18 (9): 472–479. doi:10.1016/S0168-9525(02)02744-0. ISSN 0168-9525. PMID 12175808.
- ^ Varki, Ajit; Altheide, Tasha K. (December 2005). "Comparing the human and chimpanzee genomes: searching for needles in a haystack". Genome Research. 15 (12): 1746–1758. doi:10.1101/gr.3737405. ISSN 1088-9051. PMID 16339373.
- ^ a b Cavalier-Smith, Thomas (29 June 2006). "Cell evolution and Earth history: stasis and revolution". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 969–1006. doi:10.1098/rstb.2006.1842. ISSN 0962-8436. PMC 1578732. PMID 16754610.
- ^ Schopf, J. William (29 June 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 869–885. doi:10.1098/rstb.2006.1834. ISSN 0962-8436. PMC 1578735. PMID 16754604.
- ^ Schopf, J. William (19 July 1994). "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 6735–6742. Bibcode:1994PNAS...91.6735S. doi:10.1073/pnas.91.15.6735. ISSN 0027-8424. PMC 44277. PMID 8041691.
- ^ Poole, Anthony M.; Penny, David (January 2007). "Evaluating hypotheses for the origin of eukaryotes". BioEssays. 29 (1): 74–84. doi:10.1002/bies.20516. ISSN 0265-9247. PMID 17187354.
- ^ a b Dyall, Sabrina D.; Brown, Mark T.; Johnson, Patricia J. (9 April 2004). "Ancient Invasions: From Endosymbionts to Organelles". Science. 304 (5668): 253–257. Bibcode:2004Sci...304..253D. doi:10.1126/science.1094884. ISSN 0036-8075. PMID 15073369.
- ^ Martin, William (October 2005). "The missing link between hydrogenosomes and mitochondria". Trends in Microbiology. 13 (10): 457–459. doi:10.1016/j.tim.2005.08.005. ISSN 0966-842X. PMID 16109488.
- ^ Lang, B. Franz; Gray, Michael W.; Burger, Gertraud (December 1999). "Mitochondrial genome evolution and the origin of eukaryotes". Annual Review of Genetics. 33: 351–397. doi:10.1146/annurev.genet.33.1.351. ISSN 0066-4197. PMID 10690412.
- ^ DeLong, Edward F.; Pace, Norman R. (1 August 2001). "Environmental Diversity of Bacteria and Archaea". Systematic Biology. 50 (4): 470–478. CiteSeerX 10.1.1.321.8828. doi:10.1080/106351501750435040. ISSN 1063-5157. PMID 12116647.
- ^ Kaiser, Dale (December 2001). "Building a multicellular organism". Annual Review of Genetics. 35: 103–123. doi:10.1146/annurev.genet.35.102401.090145. ISSN 0066-4197. PMID 11700279.
- ^ Zimmer, Carl (7 January 2016). "Genetic Flip Helped Organisms Go From One Cell to Many". The New York Times. Retrieved 7 January 2016.
- ^ Valentine, James W.; Jablonski, David; Erwin, Douglas H. (1 March 1999). "Fossils, molecules and embryos: new perspectives on the Cambrian explosion". Development. 126 (5): 851–859. ISSN 0950-1991. PMID 9927587. Retrieved 30 December 2014.
- ^ Ohno, Susumu (January 1997). "The reason for as well as the consequence of the Cambrian explosion in animal evolution". Journal of Molecular Evolution. 44 (Suppl. 1): S23–S27. Bibcode:1997JMolE..44S..23O. doi:10.1007/PL00000055. ISSN 0022-2844. PMID 9071008.
- Valentine, James W.; Jablonski, David (2003). "Morphological and developmental macroevolution: a paleontological perspective". The International Journal of Developmental Biology. 47 (7–8): 517–522. ISSN 0214-6282. PMID 14756327. Retrieved 30 December 2014.
- ^ Wellman, Charles H.; Osterloff, Peter L.; Mohiuddin, Uzma (2003). "Fragments of the earliest land plants" (PDF). Nature. 425 (6955): 282–285. Bibcode:2003Natur.425..282W. doi:10.1038/nature01884. PMID 13679913.
- ^ Barton, Nicholas (2007). Evolution. pp. 273–274. ISBN 9780199226320. Retrieved 30 September 2012.
- ^ Waters, Elizabeth R. (December 2003). "Molecular adaptation and the origin of land plants". Molecular Phylogenetics and Evolution. 29 (3): 456–463. doi:10.1016/j.ympev.2003.07.018. ISSN 1055-7903. PMID 14615186.
- ^ Mayhew, Peter J. (August 2007). "Why are there so many insect species? Perspectives from fossils and phylogenies". Biological Reviews. 82 (3): 425–454. doi:10.1111/j.1469-185X.2007.00018.x. ISSN 1464-7931. PMID 17624962.
- ^ Carroll, Robert L. (May 2007). "The Palaeozoic Ancestry of Salamanders, Frogs and Caecilians". Zoological Journal of the Linnean Society. 150 (Supplement s1): 1–140. doi:10.1111/j.1096-3642.2007.00246.x. ISSN 1096-3642.
- ^ Wible, John R.; Rougier, Guillermo W.; Novacek, Michael J.; Asher, Robert J. (21 June 2007). "Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary". Nature. 447 (7147): 1003–1006. Bibcode:2007Natur.447.1003W. doi:10.1038/nature05854. ISSN 0028-0836. PMID 17581585.
- ^ Witmer, Lawrence M. (28 July 2011). "Palaeontology: An icon knocked from its perch". Nature. 475 (7357): 458–459. doi:10.1038/475458a. ISSN 0028-0836. PMID 21796198.
- ^ Schloss, Patrick D.; Handelsman, Jo (December 2004). "Status of the Microbial Census". Microbiology and Molecular Biology Reviews. 68 (4): 686–691. doi:10.1128/MMBR.68.4.686-691.2004. ISSN 1092-2172. PMC 539005. PMID 15590780.
- ^ Miller & Spoolman 2012, p. 62
- ^ Mora, Camilo; Tittensor, Derek P.; Adl, Sina; et al. (23 August 2011). "How Many Species Are There on Earth and in the Ocean?". PLoS Biology. 9 (8): e1001127. doi:10.1371/journal.pbio.1001127. ISSN 1545-7885. PMC 3160336. PMID 21886479.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Bar-On YM, Phillips R and Milo R (2018) The biomass distribution on Earth PNAS, 115 (25): 6506–6511. doi:10.1073/pnas.1711842115
- ^ Madigan M; Martinko J, eds. (2006). Brock Biology of Microorganisms (13th ed.). Pearson Education. p. 1096. ISBN 978-0-321-73551-5.
- ^ Rybicki EP (1990). "The classification of organisms at the edge of life, or problems with virus systematics". South African Journal of Science. 86: 182–6. ISSN 0038-2353.
- ^ Lwoff A (1956). "The concept of virus". Journal of General Microbiology. 17 (2): 239–53. doi:10.1099/00221287-17-2-239. PMID 13481308.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ 2002 WHO mortality data Accessed 20 January 2007
- ^ "Functions of global ocean microbiome key to understanding environmental changes". www.sciencedaily.com. University of Georgia. 10 December 2015. Retrieved 11 December 2015.
- ^ Suttle, C.A. (2005). "Viruses in the Sea". Nature. 437 (9): 356–361. Bibcode:2005Natur.437..356S. doi:10.1038/nature04160. PMID 16163346.
- ^ Shors p. 5
- ^ Shors p. 593
- ^ a b c Suttle CA. Marine viruses—major players in the global ecosystem. Nature Reviews Microbiology. 2007;5(10):801–12. doi:10.1038/nrmicro1750. PMID 17853907.
- ^ Living Bacteria Are Riding Earth’s Air Currents Smithsonian Magazine, 11 January 2016.
- ^ Robbins, Jim (13 April 2018). "Trillions Upon Trillions of Viruses Fall From the Sky Each Day". The New York Times. Retrieved 14 April 2018.
- ^ Reche, Isabel; D’Orta, Gaetano; Mladenov, Natalie; Winget, Danielle M; Suttle, Curtis A (29 January 2018). "Deposition rates of viruses and bacteria above the atmospheric boundary layer". ISME Journal. 12 (4): 1154–1162. doi:10.1038/s41396-017-0042-4. PMC 5864199. PMID 29379178. Retrieved 14 April 2018.
- ^ Staff (2014). "The Biosphere". Aspen Global Change Institute. Retrieved 10 November 2014.
- ^ Takamia; et al. (1997). "Microbial flora in the deepest sea mud of the Mariana Trench". FEMS Microbiology Letters. 152 (2): 279–285. doi:10.1111/j.1574-6968.1997.tb10440.x.
- ^ National Geographic, 2005
- ^ a b c Choi, Charles Q. (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. Retrieved 17 March 2013.
- ^ Glud, Ronnie; Wenzhöfer, Frank; Middelboe, Mathias; Oguri, Kazumasa; Turnewitsch, Robert; Canfield, Donald E.; Kitazato, Hiroshi (17 March 2013). "High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth". Nature Geoscience. 6 (4): 284–288. Bibcode:2013NatGe...6..284G. doi:10.1038/ngeo1773.
- ^ Oskin, Becky (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. Retrieved 17 March 2013.
- ^ Morelle, Rebecca (15 December 2014). "Microbes discovered by deepest marine drill analysed". BBC News. Retrieved 15 December 2014.
- ^ Takai K; Nakamura K; Toki T; Tsunogai U; et al. (2008). "Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation". Proceedings of the National Academy of Sciences of the United States of America. 105 (31): 10949–54. Bibcode:2008PNAS..10510949T. doi:10.1073/pnas.0712334105. PMC 2490668. PMID 18664583.
- ^ Fox, Douglas (20 August 2014). "Lakes under the ice: Antarctica's secret garden". Nature. 512 (7514): 244–246. Bibcode:2014Natur.512..244F. doi:10.1038/512244a. PMID 25143097. Retrieved 21 August 2014.
- ^ Mack, Eric (20 August 2014). "Life Confirmed Under Antarctic Ice; Is Space Next?". Forbes. Retrieved 21 August 2014.
- ^ Wimmer E, Mueller S, Tumpey TM, Taubenberger JK. Synthetic viruses: a new opportunity to understand and prevent viral disease. Nature Biotechnology. 2009;27(12):1163–72. doi:10.1038/nbt.1593. PMID 20010599.
- ^ Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biology Direct. 2006;1:29. doi:10.1186/1745-6150-1-29. PMID 16984643.
- ^ Collier, Leslie; Balows, Albert; Sussman, Max (1998) Topley and Wilson's Microbiology and Microbial Infections ninth edition, Volume 1, Virology, volume editors: Mahy, Brian and Collier, Leslie. Arnold. Pages 33–37. ISBN 0-340-66316-2.
- ^ Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Research. 2006;117(1):156–84. doi:10.1016/j.virusres.2006.01.009. PMID 16494962.
- ^ Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. Journal of Virology. 2010;84(19):9733–48. doi:10.1128/JVI.00694-10. PMID 20660197.
- ^ In: Mahy WJ, Van Regenmortel MHV. Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 28.
- ^ a b Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H. Phage as agents of lateral gene transfer. Current Opinion in Microbiology. 2003;6(4):417–24. doi:10.1016/S1369-5274(03)00086-9. PMID 12941415.
- ^ Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question. Studies in History and Philosophy of Biological and Biomedical Sciences. 2016. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- ^ Koonin, E. V.; Starokadomskyy, P. (7 March 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Biological and Biomedical Sciences. 59: 125–34. doi:10.1016/j.shpsc.2016.02.016. PMC 5406846. PMID 26965225.
- ^ Rybicki, EP. The classification of organisms at the edge of life, or problems with virus systematics. South African Journal of Science. 1990;86:182–186.
- ^ a b Mann, NH (17 May 2005). "The Third Age of Phage". PLoS Biology. 3 (5): 753–755. doi:10.1371/journal.pbio.0030182. PMC 1110918. PMID 15884981.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews. 2000;64(1):69–114. doi:10.1128/MMBR.64.1.69-114.2000. PMID 10704475.
- ^ Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi:10.1038/nature04160. PMID 16163346.
- ^ Bergh O, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340(6233):467–8. doi:10.1038/340467a0. PMID 2755508. Bibcode:1989Natur.340..467B.
- ^ Wigington CH, Sonderegger D, Brussaard CPD, Buchan A, Finke JF, Fuhrman JA, Lennon JT, Middelboe M, Suttle CA, Stock C, Wilson WH, Wommack KE, Wilhelm SW, Weitz JS. Re-examination of the relationship between marine virus and microbial cell abundances. Nature Microbiology. 2016;1:15024. doi:10.1038/nmicrobiol.2015.24. PMID 27572161.
- ^ Krupovic M, Bamford DH (2007). "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria". BMC Genomics. 8: 236. doi:10.1186/1471-2164-8-236. PMC 1950889. PMID 17634101.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Xue H, Xu Y, Boucher Y, Polz MF (2012). "High frequency of a novel filamentous phage, VCY φ, within an environmental Vibrio cholerae population". Applied and Environmental Microbiology. 78 (1): 28–33. doi:10.1128/AEM.06297-11. PMC 3255608. PMID 22020507.
- ^ Roux S, Krupovic M, Poulet A, Debroas D, Enault F (2012). "Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads". PLOS ONE. 7 (7): e40418. Bibcode:2012PLoSO...740418R. doi:10.1371/journal.pone.0040418. PMC 3394797. PMID 22808158.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Suttle CA. Viruses in the sea. Nature. 2005;437(7057):356–61. doi:10.1038/nature04160. PMID 16163346. Bibcode:2005Natur.437..356S.
- ^ www.cdc.gov. Harmful Algal Blooms: Red Tide: Home [Retrieved 2014-12-19].
- ^ Lawrence CM, Menon S, Eilers BJ, et al.. Structural and functional studies of archaeal viruses. Journal of Biological Chemistry. 2009;284(19):12599–603. doi:10.1074/jbc.R800078200. PMID 19158076.
- ^ Prangishvili D, Forterre P, Garrett RA. Viruses of the Archaea: a unifying view. Nature Reviews Microbiology. 2006;4(11):837–48. doi:10.1038/nrmicro1527. PMID 17041631.
- ^ Prangishvili D, Garrett RA. Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses. Biochemical Society Transactions. 2004;32(Pt 2):204–8. doi:10.1042/BST0320204. PMID 15046572.
- ^ Forterre P, Philippe H. The last universal common ancestor (LUCA), simple or complex?. The Biological Bulletin. 1999;196(3):373–5; discussion 375–7. doi:10.2307/1542973. PMID 11536914.
- ^ Fredrickson JK, Zachara JM, Balkwill DL, Kennedy D, Li SM, Kostandarithes HM, Daly MJ, Romine MF, Brockman FJ (2004). "Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington state". Applied and Environmental Microbiology. 70 (7): 4230–41. doi:10.1128/AEM.70.7.4230-4241.2004. PMC 444790. PMID 15240306.
- ^ Woese CR, Kandler O, Wheelis ML (1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–9. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- ^ Schopf JW (1994). "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 6735–42. Bibcode:1994PNAS...91.6735S. doi:10.1073/pnas.91.15.6735. PMC 44277. PMID 8041691.
- ^ DeLong EF, Pace NR (2001). "Environmental diversity of bacteria and archaea". Systematic Biology. 50 (4): 470–8. CiteSeerX 10.1.1.321.8828. doi:10.1080/106351501750435040. PMID 12116647.
- ^ Brown JR, Doolittle WF (1997). "Archaea and the prokaryote-to-eukaryote transition". Microbiology and Molecular Biology Reviews. 61 (4): 456–502. PMC 232621. PMID 9409149.
- ^ Poole AM, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes". BioEssays. 29 (1): 74–84. doi:10.1002/bies.20516. PMID 17187354.
- ^ Lang BF, Gray MW, Burger G (1999). "Mitochondrial genome evolution and the origin of eukaryotes". Annual Review of Genetics. 33: 351–97. doi:10.1146/annurev.genet.33.1.351. PMID 10690412.
- ^ McFadden GI (1999). "Endosymbiosis and evolution of the plant cell". Current Opinion in Plant Biology. 2 (6): 513–9. doi:10.1016/S1369-5266(99)00025-4. PMID 10607659.
- ^ Patrick J. Keeling (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- ^ "The largest Bacterium: Scientist discovers new bacterial life form off the African coast", Max Planck Institute for Marine Microbiology, 8 April 1999, archived from the original on 20 January 2010
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ List of Prokaryotic names with Standing in Nomenclature - Genus Thiomargarita, archived from the original on 28 August 2009
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Bang C, Schmitz RA (2015). "Archaea associated with human surfaces: not to be underestimated". FEMS Microbiology Reviews. 39 (5): 631–48. doi:10.1093/femsre/fuv010. PMID 25907112.
- ^ Archaea Online Etymology Dictionary. Retrieved 17 August 2016.
- ^ Pace NR (May 2006). "Time for a change". Nature. 441 (7091): 289. Bibcode:2006Natur.441..289P. doi:10.1038/441289a. PMID 16710401.
- ^ Stoeckenius W (1 October 1981). "Walsby's square bacterium: fine structure of an orthogonal procaryote". Journal of Bacteriology. 148 (1): 352–60. PMC 216199. PMID 7287626.
- ^ Devreotes P (1989). "Dictyostelium discoideum: a model system for cell-cell interactions in development". Science. 245 (4922): 1054–8. Bibcode:1989Sci...245.1054D. doi:10.1126/science.2672337. PMID 2672337.
- ^ Cavalier-Smith T (December 1993). "Kingdom protozoa and its 18 phyla". Microbiological Reviews. 57 (4): 953–94. PMC 372943. PMID 8302218.
- ^ Corliss JO (1992). "Should there be a separate code of nomenclature for the protists?". BioSystems. 28 (1–3): 1–14. doi:10.1016/0303-2647(92)90003-H. PMID 1292654.
- ^ Slapeta J, Moreira D, López-García P (2005). "The extent of protist diversity: insights from molecular ecology of freshwater eukaryotes". Proceedings of the Royal Society B: Biological Sciences. 272 (1576): 2073–81. doi:10.1098/rspb.2005.3195. PMC 1559898. PMID 16191619.
- ^ Moreira D, López-García P (2002). "The molecular ecology of microbial eukaryotes unveils a hidden world" (PDF). Trends in Microbiology. 10 (1): 31–8. doi:10.1016/S0966-842X(01)02257-0. PMID 11755083.
- ^ Janet Fang (6 April 2010). "Animals thrive without oxygen at sea bottom". Nature. 464 (7290): 825. doi:10.1038/464825b. PMID 20376121.
- ^ "Briny deep basin may be home to animals thriving without oxygen". Science News. 23 September 2013.
- ^ Hyde, K.D.; E.B.J. Jones; E. Leaño; S.B. Pointing; A.D. Poonyth; L.L.P. Vrijmoed (1998). "Role of fungi in marine ecosystems". Biodiversity and Conservation. 7 (9): 1147–1161. doi:10.1023/A:1008823515157.
- ^ Kirk, P.M., Cannon, P.F., Minter, D.W. and Stalpers, J. "Dictionary of the Fungi". Edn 10. CABI, 2008
- ^ Hyde, K.D.; E.B.J. Jones (1989). "Spore attachment in marine fungi". Botanica Marina. 32 (3): 205–218. doi:10.1515/botm.1989.32.3.205.
- ^ San-Martín, A.; S. Orejanera; C. Gallardo; M. Silva; J. Becerra; R. Reinoso; M.C. Chamy; K. Vergara; J. Rovirosa (2008). "Steroids from the marine fungus Geotrichum sp". Journal of the Chilean Chemical Society. 53 (1): 1377–1378.
- ^ Jones, E.B.G., Hyde, K.D., & Pang, K.-L., eds. (2014). Freshwater fungi: and fungal-like organisms. Berlin/Boston: De Gruyter.
- ^ Jones, E.B.G.; Pang, K.-L., eds. (2012). Marine Fungi, and Fungal-like Organisms. Marine and Freshwater Botany. Berlin, Boston: De Gruyter (published August 2012). ISBN 978-3-11-026406-7. Retrieved 3 September 2015.
- ^ "Distribution and Diversity of Planktonic Fungi in the West Pacific Warm Pool". doi:10.1371/journal.pone.0101523.s001.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: unflagged free DOI (link) - ^ Wang, G.; Wang, X.; Liu, X.; Li, Q. (2012). "Diversity and biogeochemical function of planktonic fungi in the ocean". In Raghukumar, C. (ed.). Biology of marine fungi. Berlin, Heidelberg: Springer-Verlag. pp. 71–88. doi:10.1007/978-3-642-23342-5. ISBN 978-3-642-23341-8. Retrieved 3 September 2015.
- ^ Damare, Samir; Raghukumar, Chandralata (11 November 2007). "Fungi and Macroaggregation in Deep-Sea Sediments". Microbial Ecology. 56 (1): 168–177. doi:10.1007/s00248-007-9334-y. ISSN 0095-3628. PMID 17994287.
- ^ Kubanek, Julia; Jensen, Paul R.; Keifer, Paul A.; Sullards, M. Cameron; Collins, Dwight O.; Fenical, William (10 June 2003). "Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi". Proceedings of the National Academy of Sciences. 100 (12): 6916–6921. Bibcode:2003PNAS..100.6916K. doi:10.1073/pnas.1131855100. ISSN 0027-8424. PMC 165804. PMID 12756301.
- ^ a b Gao, Zheng; Johnson, Zackary I.; Wang, Guangyi (30 July 2009). "Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters". The ISME Journal. 4 (1): 111–120. doi:10.1038/ismej.2009.87. ISSN 1751-7362. PMID 19641535.
- ^ Panzer, Katrin; Yilmaz, Pelin; Weiß, Michael; Reich, Lothar; Richter, Michael; Wiese, Jutta; Schmaljohann, Rolf; Labes, Antje; Imhoff, Johannes F. (30 July 2015). "Identification of Habitat-Specific Biomes of Aquatic Fungal Communities Using a Comprehensive Nearly Full-Length 18S rRNA Dataset Enriched with Contextual Data". PLoS ONE. 10 (7): e0134377. Bibcode:2015PLoSO..1034377P. doi:10.1371/journal.pone.0134377. PMC 4520555. PMID 26226014.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Gutierrez, Marcelo H; Pantoja, Silvio; Quinones, Renato a and Gonzalez, Rodrigo R. First record of flamentous fungi in the coastal upwelling ecosystem off central Chile. Gayana (Concepc.) [online]. 2010, vol.74, n.1, pp. 66-73. ISSN 0717-6538.
- ^ a b Sridhar, K.R. (2009). "10. Aquatic fungi – Are they planktonic?". Plankton Dynamics of Indian Waters. Jaipur, India: Pratiksha Publications. pp. 133–148.
- ^ Species of Higher Marine Fungi Archived 22 April 2013 at the Wayback Machine University of Mississippi. Retrieved 2012-02-05.
- ^ Freshwater and marine lichen-forming fungi Retrieved 2012-02-06.
- ^ Yuan X, Xiao S (2005). "Lichen-Like Symbiosis 600 Million Years Ago". Science. 308 (5724): 1017–1020. Bibcode:2005Sci...308.1017Y. doi:10.1126/science.1111347. PMID 15890881.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Jones, E. B. Gareth; Pang, Ka-Lai (31 August 2012). Marine Fungi: and Fungal-like Organisms. Walter de Gruyter. ISBN 9783110264067.
- ^ Davidson, Michael W. (26 May 2005). "Animal Cell Structure". Molecular Expressions. Tallahassee, Fla.: Florida State University. Retrieved 3 September 2008.
- ^ Vogel, Gretchen (20 September 2018). "This fossil is one of the world's earliest animals, according to fat molecules preserved for a half-billion years". Science. AAAS. Retrieved 21 September 2018.
- ^ Bobrovskiy, Ilya (2018). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. doi:10.1126/science.aat7228. PMID 30237355.
- ^ Retallack, G.J. (2007). "Growth, decay and burial compaction of Dickinsonia, an iconic Ediacaran fossil" (PDF). Alcheringa: An Australasian Journal of Palaeontology. 31 (3): 215–240. doi:10.1080/03115510701484705.
- ^ Sperling, Erik; Vinther, Jakob; Pisani, Davide; Peterson, Kevin (2008). "A placozoan affinity for Dickinsonia and the evolution of Late Precambrian metazoan feeding modes" (PDF). In Cusack, M.; Owen, A.; Clark, N. (eds.). Programme with Abstracts. Palaeontological Association Annual Meeting. Vol. 52. Glasgow, UK. p. 81.
- ^ Gold, D. A.; Runnegar, B.; Gehling, J. G.; Jacobs, D. K. (2015). "Ancestral state reconstruction of ontogeny supports a bilaterian affinity for Dickinsonia". Evolution & Development. 17 (6): 315–397. doi:10.1111/ede.12168. PMID 26492825.
- ^ Jun-Yuan Chen; Oliveri, Paola; Feng Gao; et al. (1 August 2002). "Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China" (PDF). Developmental Biology. 248 (1): 182–196. doi:10.1006/dbio.2002.0714. ISSN 0012-1606. PMID 12142030. Archived from the original (PDF) on 26 May 2013. Retrieved 4 February 2015.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Grazhdankin, Dima (June 2004). "Patterns of distribution in the Ediacaran biotas: facies versus biogeography and evolution". Paleobiology. 30 (2): 203–221. doi:10.1666/0094-8373(2004)030<0203:PODITE>2.0.CO;2. ISSN 0094-8373.
{{cite journal}}: Invalid|ref=harv(help) - ^ Seilacher, Adolf (August 1992). "Vendobionta and Psammocorallia: lost constructions of Precambrian evolution". Journal of the Geological Society. 149 (4): 607–613. Bibcode:1992JGSoc.149..607S. doi:10.1144/gsjgs.149.4.0607. ISSN 0016-7649. Retrieved 4 February 2015.
{{cite journal}}: Invalid|ref=harv(help) - ^ Martin, Mark W.; Grazhdankin, Dmitriy V.; Bowring, Samuel A.; et al. (5 May 2000). "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution". Science. 288 (5467): 841–845. Bibcode:2000Sci...288..841M. doi:10.1126/science.288.5467.841. ISSN 0036-8075. PMID 10797002. Retrieved 5 February 2015.
{{cite journal}}: Invalid|ref=harv(help) - ^ Fedonkin, Mikhail A.; Waggoner, Benjamin M. (28 August 1997). "The late Precambrian fossil Kimberella is a mollusc-like bilaterian organism". Nature. 388 (6645): 868–871. Bibcode:1997Natur.388..868F. doi:10.1038/42242. ISSN 0028-0836.
{{cite journal}}: Invalid|ref=harv(help) - ^ Mooi, Rich; David, Bruno (December 1998). "Evolution Within a Bizarre Phylum: Homologies of the First Echinoderms" (PDF). American Zoologist. 38 (6): 965–974. doi:10.1093/icb/38.6.965. ISSN 1540-7063. Retrieved 5 February 2015.
{{cite journal}}: Invalid|ref=harv(help) - ^ McMenamin, Mark A. S. (September 2003). Spriggina is a trilobitoid ecdysozoan. Geoscience Horizons Seattle 2003. Abstracts with Programs. Vol. 35. Boulder, Colo.: Geological Society of America. p. 105. OCLC 249088612. Retrieved 24 November 2007.
{{cite conference}}: Invalid|ref=harv(help) Paper No. 40-2 presented at the Geological Society of America's 2003 Seattle Annual Meeting (2–5 November 2003) on 2 November 2003, at the Washington State Convention Center. - ^ Jih-Pai Lin; Gon, Samuel M., III; Gehling, James G.; et al. (2006). "A Parvancorina-like arthropod from the Cambrian of South China". Historical Biology: An International Journal of Paleobiology. 18 (1): 33–45. doi:10.1080/08912960500508689. ISSN 1029-2381.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Butterfield, Nicholas J. (December 2006). "Hooking some stem-group 'worms': fossil lophotrochozoans in the Burgess Shale". BioEssays. 28 (12): 1161–1166. doi:10.1002/bies.20507. ISSN 0265-9247. PMID 17120226.
{{cite journal}}: Invalid|ref=harv(help) - ^ a b Bengtson 2004, pp. 67–78
- ^ Gould, Stephen Jay (1990) Wonderful Life: The Burgess Shale and the Nature of History W. W. Norton. ISBN 9780393307009.
- ^ Valentine, James W. (2004). On the Origin of Phyla. Chicago: University Of Chicago Press. p. 7. ISBN 978-0-226-84548-7.
Classifications of organisms in hierarchical systems were in use by the seventeenth and eighteenth centuries. Usually organisms were grouped according to their morphological similarities as perceived by those early workers, and those groups were then grouped according to their similarities, and so on, to form a hierarchy.
{{cite book}}: Invalid|ref=harv(help) - ^ a b Valentine, James W (18 June 2004). On the Origin of Phyla. ISBN 9780226845487.
- ^ Erwin, Douglas; Valentine, James; Jablonski, David (1997). "Recent fossil finds and new insights into animal development are providing fresh perspectives on the riddle of the explosion of animals during the Early Cambrian". American Scientist (March–April).
- ^ a b Budd, G.E.; Jensen, S. (May 2000). "A critical reappraisal of the fossil record of the bilaterian phyla". Biological Reviews. 75 (2): 253–295. doi:10.1111/j.1469-185X.1999.tb00046.x. PMID 10881389.
- ^ Gould 1989
- ^ Budd, Graham E. (February 2003). "The Cambrian Fossil Record and the Origin of the Phyla" (PDF). Integrative and Comparative Biology. 43 (1): 157–165. doi:10.1093/icb/43.1.157. ISSN 1557-7023. PMID 21680420. Retrieved 6 February 2015.
{{cite journal}}: Invalid|ref=harv(help) - ^ Budd, Graham E. (March 1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia. 29 (1): 1–14. doi:10.1111/j.1502-3931.1996.tb01831.x. ISSN 0024-1164.
{{cite journal}}: Invalid|ref=harv(help) - ^ Marshall, Charles R. (May 2006). "Explaining the Cambrian 'Explosion' of Animals". Annual Review of Earth and Planetary Sciences. 34: 355–384. Bibcode:2006AREPS..34..355M. doi:10.1146/annurev.earth.33.031504.103001. ISSN 1545-4495.
{{cite journal}}: Invalid|ref=harv(help) - ^ a b Ponder, W.F.; Lindberg, D.R., eds. (2008). Phylogeny and Evolution of the Mollusca. Berkeley: University of California Press. p. 481. ISBN 978-0-520-25092-5.
- ^ Ruppert, Front endpaper 1
- ^ Porifera (n.) Online Etymology Dictionary. Retrieved 18 August 2016.
- ^ Vacelet & Duport 2004, pp. 179–190.
- ^ "Spongia Linnaeus, 1759". World Register of Marine Species. Retrieved 18 July 2012.
- ^ Rowland, S. M.; Stephens, T. (2001). "Archaeocyatha: A history of phylogenetic interpretation". Journal of Paleontology. 75 (6): 1065–1078. doi:10.1666/0022-3360(2001)075<1065:AAHOPI>2.0.CO;2. JSTOR 1307076. Archived from the original on 6 December 2008.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help); Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Sperling, E. A.; Pisani, D.; Peterson, K. J. (1 January 2007). "Poriferan paraphyly and its implications for Precambrian palaeobiology" (PDF). Geological Society, London, Special Publications. 286 (1): 355–368. Bibcode:2007GSLSP.286..355S. doi:10.1144/SP286.25. Archived from the original (PDF) on 20 December 2009. Retrieved 22 August 2012.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic richness" (PDF). Zootaxa. 3148: 7–12.
- ^ "Nematostella vectensis v1.0". Genome Portal. University of California. Retrieved 19 January 2014.
- ^ "Nematostella". Nematostella.org. Retrieved 18 January 2014.
- ^ a b Genikhovich, G.; U. Technau (2009). "The Starlet sea anemone Nematostella vectensis: An anthozoan model organism for studies in comparative genomics and functional evolutionary developmental biology". Cold Spring Harbor Protocols. 2009 (9): pdb.emo129. doi:10.1101/pdb.emo129. PMID 20147257.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ "Where Does Our Head Come From? Brainless Sea Anemone Sheds New Light on the Evolutionary Origin of the Head". Science Daily. 12 February 2013. Retrieved 18 January 2014.
- ^ Sinigaglia, C.; et al. (2013). "The bilaterian head patterning gene six3/6 controls aboral domain development in a cnidarian". PLoS Biology. 11 (2): e1001488. doi:10.1371/journal.pbio.1001488. PMC 3586664. PMID 23483856.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Karleskint G, Richard Turner R and, James Small J (2012) Introduction to Marine Biology Cengage Learning, edition 4, page 445. ISBN 9781133364467.
- ^ Michael Le Page (March 2019). "Animal with an anus that comes and goes could reveal how ours evolved". New Scientist.
- ^ Bavestrello, Giorgio; Christian Sommer; Michele Sarà (1992). "Bi-directional conversion in Turritopsis nutricula (Hydrozoa)". Scientia Marina. 56 (2–3): 137–140.
- ^ Piraino, Stefano; F. Boero; B. Aeschbach; V. Schmid (1996). "Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa)". Biological Bulletin. 190 (3): 302–312. doi:10.2307/1543022. JSTOR 1543022. PMID 29227703.
- ^ "Cornwall – Nature – Superstar Worm". BBC.
- ^ Mark Carwardine (1995) The Guinness Book of Animal Records. Guinness Publishing. p. 232.
- ^ "The Persistent Parasites". Time. 8 April 1957.
- ^ Hargis, William J. (1985). "Parasitology and pathology of marine organisms of the world ocean". National Oceanic and Atmospheric Administration.
{{cite journal}}: Cite journal requires|journal=(help) - ^ http://plpnemweb.ucdavis.edu/nemaplex/General/animpara.htm
- ^ Lynne S. Garcia (1999). "Classification of Human Parasites, Vectors, and Similar Organisms" (PDF). Clin Infect Dis. 29 (4): 734–6. doi:10.1086/520425. PMID 10589879.
- ^ Hodda, M (2011). "Phylum Nematoda Cobb, 1932. In: Zhang, Z.-Q. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness". Zootaxa. 3148: 63–95.
- ^ Zhang, Z (2013). "Animal biodiversity: An update of classification and diversity in 2013. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013)". Zootaxa. 3703 (1): 5–11. doi:10.11646/zootaxa.3703.1.3.
- ^ Lambshead PJD (1993). "Recent developments in marine benthic biodiversity research". Oceanis. 19 (6): 5–24.
- ^ Borgonie G, García-Moyano A, Litthauer D, Bert W, Bester A, van Heerden E, Möller C, Erasmus M, Onstott TC (June 2011). "Nematoda from the terrestrial deep subsurface of South Africa". Nature. 474 (7349): 79–82. Bibcode:2011Natur.474...79B. doi:10.1038/nature09974. PMID 21637257.
- ^ Danovaro R, Gambi C, Dell'Anno A, Corinaldesi C, Fraschetti S, Vanreusel A, Vincx M, Gooday AJ (January 2008). "Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss". Current Biology. 18 (1): 1–8. doi:10.1016/j.cub.2007.11.056. PMID 18164201.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Platt HM (1994). "foreword". In Lorenzen S, Lorenzen SA (eds.). The phylogenetic systematics of freeliving nematodes. London: The Ray Society. ISBN 978-0-903874-22-9.
- ^ "Sea Lily". Science Encyclopedia. Retrieved 5 September 2014.
- ^ "Animal Diversity Web - Echinodermata". University of Michigan Museum of Zoology. Retrieved 26 August 2012.
- ^ Fox, Richard. "Asterias forbesi". Invertebrate Anatomy OnLine. Lander University. Retrieved 14 June 2014.
- ^ Holsinger, K. (2005). Keystone species. Retrieved 10 May 2010, from "Archived copy". Archived from the original on 30 June 2010. Retrieved 12 May 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Chapman, A.D. (2009). Numbers of Living Species in Australia and the World, 2nd edition. Australian Biological Resources Study, Canberra. Retrieved 12 January 2010. ISBN 978-0-642-56860-1 (printed); ISBN 978-0-642-56861-8 (online).
- ^ Hancock, Rebecca (2008). "Recognising research on molluscs". Australian Museum. Archived from the original on 30 May 2009. Retrieved 9 March 2009.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Barnes, R.S.K.; Calow, P.; Olive, P.J.W.; Golding, D.W.; Spicer, J.I. (2001). The Invertebrates, A Synthesis (3rd ed.). UK: Blackwell Science.
{{cite book}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ "Welcome to CephBase". CephBase. Retrieved 29 January 2016.
- ^ Wilbur, Karl M.; Clarke, M.R.; Trueman, E.R., eds. (1985), The Mollusca, vol. 12. Paleontology and neontology of Cephalopods, New York: Academic Press, ISBN 0-12-728702-7
- ^ "Are there any freshwater cephalopods?". 16 January 2013.
- ^ Black, Richard (26 April 2008). "Colossal squid out of the freezer". BBC News.
- ^ Ewen Callaway (2 June 2008). "Simple-Minded Nautilus Shows Flash of Memory". New Scientist. Retrieved 7 March 2012.
- ^ Kathryn Phillips (15 June 2008). "Living Fossil Memories" (PDF). Journal of Experimental Biology. 211 (12): iii. doi:10.1242/jeb.020370.
- ^ Robyn Crook; Jennifer Basil (2008). "A biphasic memory curve in the chambered nautilus, Nautilus pompilius L. (Cephalopoda: Nautiloidea)" (PDF). Journal of Experimental Biology. 211 (12): 1992–1998. doi:10.1242/jeb.018531. PMID 18515730.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ a b c Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology (7th ed.). Cengage Learning. ISBN 978-81-315-0104-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Hayward, PJ (1996). Handbook of the Marine Fauna of North-West Europe. Oxford University Press. ISBN 978-0-19-854055-7.
- ^ Giribet, G.; Okusu, A, A.; Lindgren, A.R., A. R.; Huff, S.W., S. W.; Schrödl, M, M.; Nishiguchi, M.K., M. K. (May 2006). "Evidence for a clade composed of molluscs with serially repeated structures: monoplacophorans are related to chitons" (Free full text). Proceedings of the National Academy of Sciences of the United States of America. 103 (20): 7723–7728. Bibcode:2006PNAS..103.7723G. doi:10.1073/pnas.0602578103. PMC 1472512. PMID 16675549.
- ^ Healy, J.M. (2001). "The Mollusca". In Anderson, D.T. (ed.). Invertebrate Zoology (2nd ed.). Oxford University Press. pp. 120–171. ISBN 978-0-19-551368-4.
- ^ Ruppert, Fox & Barnes (2004), pp. 518–522
- ^ "Archived copy". Archived from the original on 26 January 2011. Retrieved 10 March 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Braddy, Simon J.; Poschmann, Markus; Tetlie, O. Erik (2007). "Giant claw reveals the largest ever arthropod". Biology Letters. 4 (1): 106–109. doi:10.1098/rsbl.2007.0491. PMC 2412931. PMID 18029297.
- ^ Cressey, Daniel (21 November 2007). "Giant sea scorpion discovered". Nature. doi:10.1038/news.2007.272. Retrieved 10 June 2013.
- ^ Patrick Kilday (28 September 2005). "Mantis shrimp boasts most advanced eyes". The Daily Californian.
- ^ S. N. Patek; R. L. Caldwell (2005). "Extreme impact and cavitation forces of a biological hammer: strike forces of the peacock mantis shrimp". Journal of Experimental Biology. 208 (19): 3655–3664. doi:10.1242/jeb.01831. PMID 16169943.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ D. R. Currie; T. M. Ward (2009). South Australian Giant Crab (Pseudocarcinus gigas) Fishery (PDF). South Australian Research and Development Institute. Fishery Assessment Report for PIRSA. Retrieved 9 December 2013.
{{cite book}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ "An ugly giant crab of Japan". Popular Science. 96 (6): 42. 1920.
- ^ Gewin, V (2005). "Functional genomics thickens the biological plot". PLoS Biology. 3 (6): e219. doi:10.1371/journal.pbio.0030219. PMC 1149496. PMID 15941356.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lancelet (amphioxus) genome and the origin of vertebrates Ars Technica, 19 June 2008.
- ^ Lemaire, P (2011). "Evolutionary crossroads in developmental biology: the tunicates". Development. 138 (11): 2143–2152. doi:10.1242/dev.048975. PMID 21558365.
- ^ "FishBase: A Global Information System on Fishes". FishBase. Retrieved 17 January 2017.
- ^ "How Many Fish In The Sea? Census Of Marine Life Launches First Report". Science Daily. Retrieved 17 January 2017.
- ^ Docker, Margaret F. (2006). "Bill Beamish's Contributions to Lamprey Research and Recent Advances in the Field". Guelph Ichthyology Reviews. 7. doi:10.1111/j.1095-8649.2006.00968.x (inactive 10 February 2019).
{{cite journal}}: CS1 maint: DOI inactive as of February 2019 (link) - ^ Hardisty, M. W.; Potter, I. C. (1971). Hardisty, M. W.; Potter, I. C. (eds.). The Biology of Lampreys (1st ed.). Academic Press. ISBN 978-0-123-24801-5.
- ^ Gill, Howard S.; Renaud, Claude B.; Chapleau, François; Mayden, Richard L.; Potter, Ian C.; Douglas, M. E. (2003). "Phylogeny of Living Parasitic Lampreys (Petromyzontiformes) Based on Morphological Data". Copeia. 2003 (4): 687–703. doi:10.1643/IA02-085.1.
- ^ "Myxini". University of California Museum of Paleontology. Retrieved 17 January 2017.
- ^ Greena, Stephen A.; Bronner, Marianne E. (2014). "The Lamprey: A jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits". Differentiation. 87 (1–2): 44–51. doi:10.1016/j.diff.2014.02.001. PMC 3995830. PMID 24560767.
- ^ a b Wroe, S.; Huber, D. R.; Lowry, M.; McHenry, C.; Moreno, K.; Clausen, P.; Ferrara, T. L.; Cunningham, E.; Dean, M. N.; Summers, A. P. (2008). "Three-dimensional computer analysis of white shark jaw mechanics: how hard can a great white bite?" (PDF). Journal of Zoology. 276 (4): 336–342. doi:10.1111/j.1469-7998.2008.00494.x.
- ^ Pimiento, Catalina; Dana J. Ehret; Bruce J. MacFadden; Gordon Hubbell (10 May 2010). Stepanova, Anna (ed.). "Ancient Nursery Area for the Extinct Giant Shark Megalodon from the Miocene of Panama". PLoS ONE. 5 (5): e10552. Bibcode:2010PLoSO...510552P. doi:10.1371/journal.pone.0010552. PMC 2866656. PMID 20479893.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lambert, Olivier; Bianucci, Giovanni; Post, Klaas; de Muizon, Christian; Salas-Gismondi, Rodolfo; Urbina, Mario; Reumer, Jelle (1 July 2010). "The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru". Nature. 466 (7302): 105–108. Bibcode:2010Natur.466..105L. doi:10.1038/nature09067. PMID 20596020.
- ^ Nielsen, Julius; Hedeholm, Rasmus B.; Heinemeier, Jan; Bushnell, Peter G.; Christiansen, Jørgen S.; Olsen, Jesper; Ramsey, Christopher Bronk; Brill, Richard W.; Simon, Malene; Steffensen, Kirstine F.; Steffensen, John F. (2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–4. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. PMID 27516602.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Black, Richard (11 June 2007). "Sawfish protection acquires teeth". BBC News.
- ^ Marshall, A.; Bennett, M.B.; Kodja, G.; Hinojosa-Alvarez, S.; Galvan-Magana, F.; Harding, M.; Stevens, G.; Kashiwagi, T. (2011). "Manta birostris". The IUCN Red List of Threatened Species. 2011: e.T198921A9108067. doi:10.2305/IUCN.UK.2011-2.RLTS.T198921A9108067.en. Retrieved 23 December 2017.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Hopkins Gareth R.; Brodie Edmund D. Jr (2015). "Occurrence of Amphibians in Saline Habitats: A Review and Evolutionary Perspective". Herpetological Monographs. 29 (1): 1–27. doi:10.1655/HERPMONOGRAPHS-D-14-00006.
- ^ Natchev, Nikolay; Tzankov, Nikolay; Geme, Richard (2011). "Green frog invasion in the Black Sea: habitat ecology of the Pelophylax esculentus complex (Anura, Amphibia) population in the region of Shablenska Тuzla lagoon in Bulgaria" (PDF). Herpetology Notes. 4: 347–351.
- ^ Modesto, S.P.; Anderson, J.S. (2004). "The phylogenetic definition of Reptilia". Systematic Biology. 53 (5): 815–821. doi:10.1080/10635150490503026. PMID 15545258.

- ^ Gauthier, J.A.; Kluge, A.G.; Rowe, T. (1988). "The early evolution of the Amniota". In Benton, M.J. (ed.). The Phylogeny and Classification of the Tetrapods. Vol. 1. Oxford: Clarendon Press. pp. 103–155. ISBN 978-0-19-857705-8.
- ^ Laurin, M.; Reisz, R. R. (1995). "A reevaluation of early amniote phylogeny" (PDF). Zoological Journal of the Linnean Society. 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x.

- ^ Modesto, S.P. (1999). "Observations of the structure of the Early Permian reptile Stereosternum tumidum Cope". Palaeontologia Africana. 35: 7–19.
- ^ Rasmussen, Arne Redsted; Murphy, John C.; Ompi, Medy; Gibbons, J. Whitfield; Uetz, Peter (8 November 2011). "Marine Reptiles". PLoS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sander, P. Martin (2012). "Reproduction in early amniotes". Science. 337 (6096): 806–808. Bibcode:2012Sci...337..806S. doi:10.1126/science.1224301. PMID 22904001.
- ^ Stidworthy J. 1974. Snakes of the World. Grosset & Dunlap Inc. 160 pp. ISBN 0-448-11856-4.
- ^ Sea snakes[permanent dead link] at Food and Agriculture Organization of the United Nations. Accessed 7 August 2007.
- ^ Rasmussen, A.R.; Murphy, J.C.; Ompi, M.; Gibbons, J.W.; Uetz, P. (2011). "Marine reptiles". PLOS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Martill D.M. (1993). "Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany". Kaupia - Darmstädter Beiträge zur Naturgeschichte, 2 : 77-97.
- ^ Gould, Stephen Jay (1993) "Bent Out of Shape" in Eight Little Piggies: Reflections in Natural History. Norton, 179–94. ISBN 9780393311396.
- ^ "Sardine Run Shark Feeding Frenzy Phenomenon in Africa". Archived from the original on 2 December 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "The Society for Marine Mammalogy's Taxonomy Committee List of Species and subspecies". Society for Marine Mammalogy. October 2015. Archived from the original on 6 January 2015. Retrieved 23 November 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Romer A. S. and Parsons T. S. (1986) The Vertebrate Body, page 96, Sanders College Publishing. ISBN 0030584469.
- ^ "Blue whale". World Wide Fund For Nature. Retrieved 15 August 2016.
- ^ Marino, Lori (2004). "Cetacean Brain Evolution: Multiplication Generates Complexity" (PDF). International Society for Comparative Psychology (17): 1–16.
- ^ Lalli, C.; Parsons, T. (1993). Biological Oceanography: An Introduction. Butterworth-Heinemann. ISBN 0 7506 3384 0.
- ^ John Dolan (November 2012). "Microzooplankton: the microscopic (micro) animals (zoo) of the plankton" (PDF).
- ^ Harvey, Edmund Newton (1952). Bioluminescence. Academic Press.
- ^ Prentice, I.C. (2001). "The carbon cycle and atmospheric carbon dioxide". Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change / Houghton, J.T. [edit.] Retrieved 31 May 2012.
- ^ DeLong, E.F.; Beja, O. (2010). "The light-driven proton pump proteorhodopsin enhances bacterial survival during tough times". PLoS Biology. 8 (4): e1000359. doi:10.1371/journal.pbio.1000359. PMC 2860490. PMID 20436957. e1000359.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; Spudich, E.N. (2000). "Bacterial rhodopsin: evidence for a new type of phototrophy in the sea". Science. 289 (5486): 1902–1906. Bibcode:2000Sci...289.1902B. doi:10.1126/science.289.5486.1902. PMID 10988064.
- ^ "Interviews with Fellows: Ed Delong". American Academy of Microbiology. Retrieved 2 July 2016.
- ^ Bacteria with Batteries, Popular Science, January 2001, Page 55.
- ^ Rosing, M.; Bird, D.; Sleep, N.; Bjerrum, C. (2010). "No climate paradox under the faint early Sun". Nature. 464 (7289): 744–747. Bibcode:2010Natur.464..744R. doi:10.1038/nature08955. PMID 20360739.
- ^ a b Sahney, S.; Benton, M.J.; Ferry, Paul (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land". Biology Letters. 6 (4): 544–7. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
- ^ McKinney 1997, p. 110
- ^ Stearns & Stearns 1999, p. x
- ^ Novacek, Michael J. (8 November 2014). "Prehistory's Brilliant Future". The New York Times. New York: The New York Times Company. ISSN 0362-4331. Retrieved 25 December 2014.
- ^ Nee, S. (2004). "Extinction, slime, and bottoms". PLoS Biology. 2 (8): E272. doi:10.1371/journal.pbio.0020272. PMC 509315. PMID 15314670.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ward, Peter D (2006). "Impact from the Deep". Scientific American. 295 (4): 64–71. Bibcode:2006SciAm.295d..64W. doi:10.1038/scientificamerican1006-64. PMID 16989482.
- ^ Sahney, S.; Benton, M.J. (2008). "Recovery from the most profound mass extinction of all time" (PDF). Proceedings of the Royal Society B: Biological Sciences. 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Desmond, Adrian (1975). "The Discovery of Marine Transgressions and the Explanation of Fossils in Antiquity". American Journal of Science. 275 (6): 692–697. Bibcode:1975AmJS..275..692D. doi:10.2475/ajs.275.6.692.
- ^ McKirahan, Richard D. "Xenophanes of Colophon. Philosophy Before Socrates. Indianapolis: Hackett Publishing Company, 1994. 66. Print.
- ^ Grene MG, Grene M and Depew D (2004) The Philosophy of Biology: An Episodic History pages 1–34, Cambridge University Press. ISBN 9780521643801.
- ^ Boylan, Michael "Aristotle: Biology" Internet Encyclopedia of Philosophy. Retrieved 5 August 2016.
- ^ Lee, H. D. P. (1948) "Place-Names and the date of Aristotle's Biological Works". Classical Quarterly, 42 (3/4) :61–7
- ^ Singer, Charles. A short history of biology. Oxford 1931.
- ^ Emily Kearns, "Animals, knowledge about," in Oxford Classical Dictionary, 3rd ed., 1996, p. 92.
- ^ Carl T. Bergstrom; Lee Alan Dugatkin (2012). Evolution. Norton. p. 35. ISBN 978-0-393-92592-0.
- ^ Rhodes, Frank Harold Trevor (1 January 1974). Evolution. Golden Press. p. 7. ISBN 978-0-307-64360-5.
- ^ a b c Lalli, Carol M., and Timothy R. Parsons. "Introduction." Biological Oceanography: An Introduction. First Edition ed. Tarrytown, New York: Pergamon, 1993. 7-21. Print.
- ^ Menden-Deuer, Susanne. "Course Info, OCG 561 Biological Oceanography".
- ^ Miller, Charles B.; Patricia A. Wheeler (2012). Biological Oceanography (Second ed.). Chinchester, West Sussex: John Wiley & Sons.
Further references
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D'Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; Fujita, R. (2008). "A global map of human impact on marine ecosystems". Science. 319 (5865): 948–952. Bibcode:2008Sci...319..948H. doi:10.1126/science.1149345. PMID 18276889.
- Paleczny, M.; Hammill, E.; Karpouzi, V.; Pauly, D. (2015). "Population trend of the world's monitored seabirds, 1950-2010". PLoS ONE. 10 (6): e0129342. Bibcode:2015PLoSO..1029342P. doi:10.1371/journal.pone.0129342. PMC 4461279. PMID 26058068.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - After 60 million years of extreme living, seabirds are crashing The Guardian, 22 September 2015.
- Ruppert, E.E.; Fox, R.S.; Barnes, R.D. (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. ISBN 978-0-03-025982-1.
{{cite book}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help)







![The chloroplasts of glaucophytes have a peptidoglycan layer, evidence suggesting their endosymbiotic origin from cyanobacteria.[126]](/upwiki/wikipedia/commons/thumb/c/c9/Glaucocystis_sp.jpg/280px-Glaucocystis_sp.jpg)

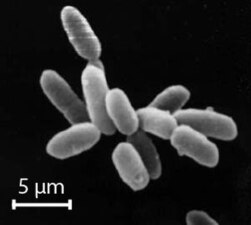


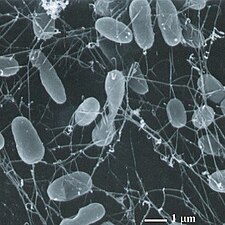

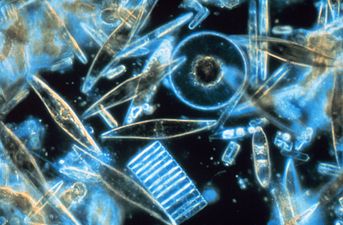

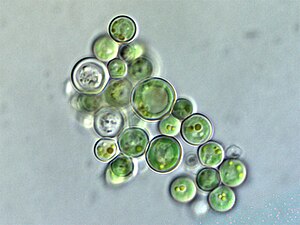

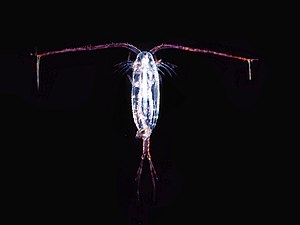
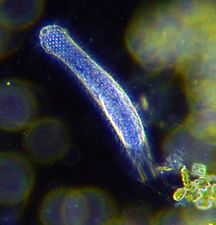
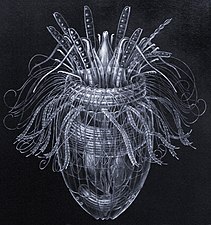

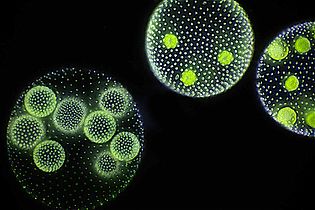












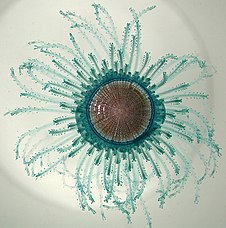
![Lion's mane jellyfish, largest known jellyfish[196]](/upwiki/wikipedia/commons/thumb/2/22/Largelionsmanejellyfish.jpg/171px-Largelionsmanejellyfish.jpg)
![The bioluminescent sea walnut has a transient anus[197] which forms only when it needs to defecate](/upwiki/wikipedia/commons/thumb/f/f0/Sea_walnut%2C_Boston_Aquarium_%28cropped%29.jpg/265px-Sea_walnut%2C_Boston_Aquarium_%28cropped%29.jpg)
![Turritopsis dohrnii achieves biological immortality by transfering its cells back to childhood [198][199]](/upwiki/wikipedia/commons/b/b0/Turritopsis_dohrnii_%28cropped%29.jpg)




![The ochre sea star was the first keystone predator to be studied. They limit mussels which can overwhelm intertidal communities.[215]](/upwiki/wikipedia/commons/thumb/8/89/Ochre_sea_star.jpg/300px-Ochre_sea_star.jpg)




![Colossal squid, largest of all invertebrates[222]](/upwiki/wikipedia/commons/thumb/3/37/Calmarcolossal.jpg/147px-Calmarcolossal.jpg)
![The nautilus is a living fossil little changed since it evolved 500 million years ago as one of the first cephalopods.[223][224][225]](/upwiki/wikipedia/commons/thumb/d/d5/Nautilus_Palau.JPG/280px-Nautilus_Palau.JPG)





![Fossil trilobite. Trilobites first appeared about 521 Ma. They were highly successful and were found everywhere in the ocean for 270 Ma.[231]](/upwiki/wikipedia/commons/thumb/a/aa/Cheirurus_ingricus.png/301px-Cheirurus_ingricus.png)
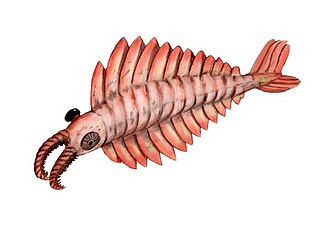
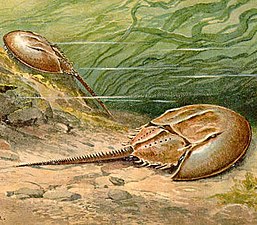
![The largest known arthropod, the sea scorpion Jaekelopterus rhenaniae, has been found in estuarine strata from about 390 Ma. It was up to 2.5 m (8.2 ft) long.[232][233]](/upwiki/wikipedia/commons/thumb/a/af/Jaekelopterus_rhenaniae_reconstruction.jpg/338px-Jaekelopterus_rhenaniae_reconstruction.jpg)

![Mantis shrimp have the most advanced eyes in the animal kingdom,[234] and smash prey by swinging their club-like raptorial claws.[235]](/upwiki/wikipedia/commons/thumb/b/b2/OdontodactylusScyllarus.jpg/298px-OdontodactylusScyllarus.jpg)
![The Tasmanian giant crab is long-lived and slow-growing, making it vulnerable to overfishing.[236]](/upwiki/wikipedia/commons/thumb/2/2c/J_J_Wild_Pseudocarcinus_cropped.jpg/330px-J_J_Wild_Pseudocarcinus_cropped.jpg)
![The Japanese spider crab has the longest leg span of any arthropod, reaching 5.5 metres (18 ft) from claw to claw.[237]](/upwiki/wikipedia/commons/thumb/a/a7/Macrocheira_kaempferi.jpg/314px-Macrocheira_kaempferi.jpg)
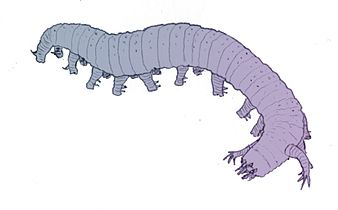

![The lancelet, a small translucent fish-like Cephalochordate, is the closest living invertebrate relative of the vertebrates.[238][239]](/upwiki/wikipedia/commons/thumb/5/54/Amphioxus.png/332px-Amphioxus.png)
![Fluorescent-colored sea squirts, Rhopalaea crassa. Tunicates may provide clues to vertebrate (and therefore human) ancestry.[240]](/upwiki/wikipedia/commons/thumb/8/8c/Ascidian_%28Rhopalaea_Crassa%29_%284_cm%29.png/280px-Ascidian_%28Rhopalaea_Crassa%29_%284_cm%29.png)

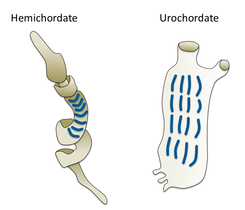

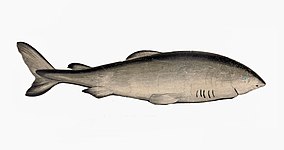



![Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[252]](/upwiki/wikipedia/commons/thumb/9/9b/Sawfish_genova.jpg/200px-Sawfish_genova.jpg)
![The giant manta ray, largest ray in the world, has been targeted by fisheries and is now vulnerable.[253]](/upwiki/wikipedia/commons/thumb/8/85/Manta_alfredi_fushivaru_thila.jpg/200px-Manta_alfredi_fushivaru_thila.jpg)

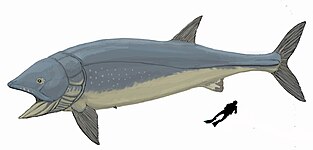









![Endangered blue whale, largest animal ever[270]](/upwiki/wikipedia/commons/thumb/c/cd/Bluewhale2_noaa.jpg/270px-Bluewhale2_noaa.jpg)
![Bottlenose dolphin, highest encephalization of any animal after humans[271]](/upwiki/wikipedia/commons/thumb/1/10/Tursiops_truncatus_01.jpg/271px-Tursiops_truncatus_01.jpg)






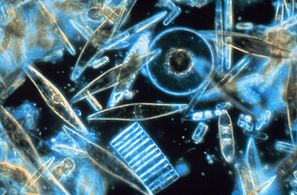



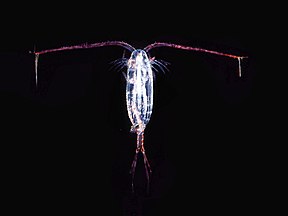

![Tomopteris, a planktonic segmented worm with unusual yellow bioluminescence[274]](/upwiki/wikipedia/commons/thumb/4/40/Tomopteriskils.jpg/318px-Tomopteriskils.jpg)


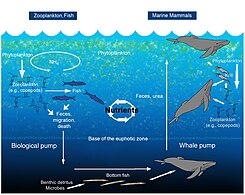
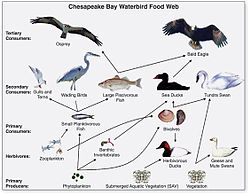
![Marine carbon cycle[275]](/upwiki/wikipedia/commons/thumb/0/03/OceanCarbonCycle.jpg/207px-OceanCarbonCycle.jpg)