User talk:CHI565-Fe/sandbox
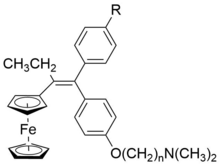 |
Ferrocifens are a family of organometallic complexes. They are ferrocenyl analogue of the antitumor agent tamoxifen and are potential drug candidates to treat certain types of tamoxifen-resistent breast cancer.[1]
Background
[edit]The standard selective estrogen receptor modulator, tamoxifen, is only effective for the estrogen receptor positive (ER(+)) breast cancer, accounting for approxiamtely 2/3 of cases.[2] The major active metabolite of tamoxifen is 4-hydroxytamoxifen, which exerts its antiproliferative effect through competitive binding to estrogen receptor. However, under long-term treatment of tamoxifen, expression of receptor tends to become down-regulated and patients might build up resistance in body.[3] Thus it is anticipated to find a multi-model drug to treat the estrogen receptor negative (ER(-)) or tamoxifen-resistant breast cancer. Also, the discovery of the chemotherapy medication cisplatin, especially its spectacular success in curing testicular cancer, has inspired numerous research to look for organometallic compounds as anti-cancer agents.[4] Different from tamoxifen, ferrocifens showed antiproliferative effect both on ER(+) and ER(-) breast-cancer cell lines.[5]
Synthetic route
[edit]
Ferrocifen was first synthesized in 1996 by Gérard Jaouen group.[6] Reaction between ferrocenyl acetic acid and organolithium CH3OC6H4Li is utilised to give the ketone. Then an ethyl group is attached at the α position of the ferrocenyl group by using a strong base t-BuOK in dimethyl sulfoxide, and followed by the addition of the electrophilic reagent ethyl iodide. The subsequent step is the addition reaction between phenyllithium and ketone, followed by the dehydration of the intermediate alcohol to synthesize ethylene skeleton. Finally, addition of (dimethylamino)ethyl chloride, hydrochloride in ethanol solution gives ferrocifen.

However, the first synthetic route is time consuming, and the overall yield from ferrocenyl acetic acid is quite low.[6] A better and convenient method is using McMurry reaction.[3] Propionylferrocene is synthesized by acylation of ferrocene and propionic anhydride via Friedel-Crafts reaction. In the presence of McMurry reagent, the propionylferrocene obtained reacts with dihydroxybenzophenone to yield the halogenated compound, which then reacts with dimethylamine chlorhydrate to give ferrocifen.
Anti-cancer mode of action
[edit]The antiproliferative effects of ferrocifens and their active metabolites hydroxyl-ferrocifens have been amply investigated on different types of breast-cancer cell lines by Gérard Jaouen et al.[4][6][7][8][9]
In the estrogen dependent ER(+) MCF-7 cell line, ferrocifens demonstrated tamoxifen-like antiestrogenic effect, which is attributed to the similar mechanism of competitive binding to estrogen receptor. Structure-activity-relationship study showed that replacement of the phenol moiety by ferrocene reduces the receptor binding affinity to 40% compared to tamoxifen.[4]
However, some ferrocifens also showed a remarkable antiproliferative activity on the MDA-MB-231 breast-cancer cell line, which belongs to the ER(-) type, thus the introduced ferrocene moiety should be responsible for this additional cytotoxicity.[4][7] By modelling the reactivity of hydroxy-ferrocifens using electrochemistry approach, a redox activation mechanism was proposed as its second mode of action, in which hydroxy-ferrocifen is readily oxidized, with the ferrocene group acting as an intramolecular reversible redox “antenna”.[4][8] The cytotoxicity is believed to arise from the subsequent generation of the quinone methide intermediate, which will be attacked and form adducts with species in cancer cells such as DNA nucleobases, glutathione or proteins, and finally lead to cell death. Moreover, reactive oxygen species were also detected in cell lines after treating with ferrocifens.[9]
References
[edit]- ^ Jaouen, Gérard; et al. (2015). "Ferrocifen type anti cancer drugs". Chem. Soc. Rev. 44: 8802--881. doi:10.1039/c5cs00486a.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ Siden, Top; et al. (2001). "Studies on organometallic selective estrogen receptor modulators.(SERMs) Dual activity in the hydroxy-ferrocifen series". J. Organomet. Chem. 637: 500-506. doi:10.1016/S0022-328X(01)00953-6.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b Siden, Top; et al. (1997). "Facile route to ferrocifen, 1-[4-(2-dimethylaminoethoxy)]-1-(phenyl-2-ferrocenyl-but-1-ene), first organometallic analogue of tamoxifen, by the McMurry reaction". J. Organomet. Chem. 541: 355-361. doi:10.1016/S0022-328X(97)00086-7.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b c d e Elizabeth, Hillard; et al. (2006). "Ferrocene‐mediated proton‐coupled electron transfer in a series of ferrocifen‐type breast‐cancer drug candidates". Angew. Chem. 45: 291-296. doi:10.1002/anie.200502925.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ Nguyen, Anh; et al. (2007). "Ferrocifens and ferrocifenols as new potential weapons against breast cancer". CHIMIA. 61: 716-724. doi:10.2533/chimia.2007.716.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b c Siden, Top; et al. (1996). "Ferrocenyl hydroxytamoxifen: a prototype for a new range of oestradiol receptor site-directed cytotoxics". ChemComm (8): 955-956. doi:10.1039/CC9960000955.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b Elizabeth, Hillard; et al. (2007). "The influence of phenolic hydroxy substitution on the electron transfer and anti-cancer properties of compounds based on the 2-ferrocenyl-1-phenyl-but-1-ene motif". Dalton Trans. (43): 5073-5081. doi:10.1039/B705030E.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b Wang, Yong; et al. (2015). "Organometallic antitumor compounds: ferrocifens as precursors to quinone methides". Angew. Chem. Int. Ed. 54: 10230-10233. doi:10.1002/anie.201503048.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ a b Domenico, Osella; et al. (2005). "FACS analysis of oxidative stress induced on tumour cells by SERMs". Inorganica Chim. Acta. 358 (6): 1993-1998. doi:10.1016/j.ica.2004.11.027.
{{cite journal}}: Explicit use of et al. in:|first1=(help)
Category:Ferrocenes Category:Sandwich compounds Category:Selective estrogen receptor modulators Category:Breast cancer
