Talk:Lactic acid
| This is the talk page for discussing improvements to the Lactic acid article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| This article has not yet been rated on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||||||||||||||||||||||
Please add the quality rating to the {{WikiProject banner shell}} template instead of this project banner. See WP:PIQA for details.
Please add the quality rating to the {{WikiProject banner shell}} template instead of this project banner. See WP:PIQA for details.
Please add the quality rating to the {{WikiProject banner shell}} template instead of this project banner. See WP:PIQA for details.
| ||||||||||||||||||||||||||||||||||||||
Text exactly matches article from Scientific American
I just coincidentally read these two articles one right after the other. It's not clear to me from some other comments on this talk page whether the material is copied with permission from the author, but I wanted to clarify that. Anyone? http://www.scientificamerican.com/article.cfm?id=why-does-lactic-acid-buil Elf | Talk 00:50, 8 January 2014 (UTC)
Clarify Lactic Acid and Lactate
hello(((((((((((((((((((:Italic text I came to this article to read up on the effects of lactate on muscle performance with a substantial background knowledge in cellular biology and chemistry. But, my concern is that the article doesn't clarify the difference between the two compounds sufficiently. It can appear confusing that half the article is discussing lactic acid in milk and other dairy products and then the other half is about lactic acid's derivative lactate and what its effects in muscles are.
I propose either splitting the articles into two or clarifying the difference better between the two compounds. --Novaprospekt 22:58, 2 May 2007 (UTC)
- I agree that it's currently unclear. I think for the moment, it would be best to just clarify it and split the article later when it gets bigger. I'll work on something over the next couple of days. Rerun 06:44, 14 May 2007 (UTC)
- After thinking about this for a while and trying to come up with a workable solution, I've changed my mind and now think that it would be best to split the article, similar to acetate and acetic acid. I think there is enough information present to warrant a separate page for each. However I don't want to rush ahead and do this. I'll wait two weeks and if no one has any objections I'll split the pages. I'm open to discussion so if anyone wants the page to stay as it is, please leave a message if you do Rerun 05:15, 22 May 2007 (UTC)
- Certainly one could focus on the biological aspects and the other on the chemical. It might work. David D. (Talk) 05:18, 22 May 2007 (UTC)
- After thinking about this for a while and trying to come up with a workable solution, I've changed my mind and now think that it would be best to split the article, similar to acetate and acetic acid. I think there is enough information present to warrant a separate page for each. However I don't want to rush ahead and do this. I'll wait two weeks and if no one has any objections I'll split the pages. I'm open to discussion so if anyone wants the page to stay as it is, please leave a message if you do Rerun 05:15, 22 May 2007 (UTC)
As it appears discussion of lactate is staying in this article, I have changed lactate from a redirect to a disambiguation page and also fixed all mainspace links to lactate. --Una Smith (talk) 04:24, 20 December 2007 (UTC)
Image
Sorry for inserting the image in such a messy manner. It looks like Wikipedia won't use the HTML I'm used to. Could someone else please straighten out the image and make it "fit" th entry?? Sorry I'm so much of an amateur at these things right now.
- Why isn't there a structural formula on the page? Space-filling models are peachy, sure, but it would be nice to have a line drawing...
Lactic build up myth
(copied from my post on DOMS) Lactic acid(actually lactate) is associated with sore muscles, but it has been shown not to be the cause of the soreness. It has been found that people with greater endurance have higher levels of lactate, and with some further experiments showed that lactate is actually beneficial for reducing soreness and protecting the muscles.
http://www.abc.net.au/catalyst/stories/s1314443.htm http://www.time-to-run.com/theabc/lactic.htm njh 23:31, 6 October 2005 (UTC)
OK- it now turns out the lactic acid is FUEL for muscles.... so this section might need an update? http://www.nytimes.com/2006/05/16/health/nutrition/16run.html?ex=1148097600&en=1d44fa0b49133c03&ei=5087%0A --spiralhighway 19:03, 18 May 2006 (UTC)
I previously posted a reference on Talk:Anaerobic_respiration with regard to this, but I haven't seen any changes to that article and a related one (with a probably factually incorrect title): Lactic_acidosis. The reference is http://ajpregu.physiology.org/cgi/reprint/287/3/R502.pdf , which states that lactate production actually consumes protons and facilitates proton removal from the cell, aiding the other buffer systems to prevent the extent of acidosis. Can any expert please confirm this, and edit these three pages? Lim Wei Quan (talk) 06:10, 30 October 2008 (UTC) [Edit] I just realised somebody has commented on this further down, but what about the other article on lactic acidosis? [/Edit]
Wrong picture
The bottom space-filling model seems to be glycine and not lactic acid.
Melting Point
Most MSDS's I have found show the melting point of lactic acid to be 18C or around 64F
- In this case, the single enantiomers crystalize differently than the racemate, so they have different melting points. I have added this info into the article. --Ed (Edgar181) 19:25, 20 March 2006 (UTC)
id add then in ""handbook of chemistry and physics" 48th edition 1967-1968 MP is listed as 25 for d 26 for l and 18 for dl — Preceding unsigned comment added by Tiphtiphy (talk • contribs) 10:20, 25 December 2018 (UTC)
Additions
I have made a fair few changes to this page as a lot of the information was outdated. I expect people will be a little concerned over the information I posted that says that we don't produce lactic acid. The best way to investigate this is to find a a picture of glycolosis and start counting the hydrogens and you'll find that it's not possible to make lactic acid under normal conditions. Also, have a read of the last external link posted for more information about this.
Strong ion difference
I reverted the part in Exercise and lactate about lactate and burning etc, to indicate that lactate does not "directly" cause acidosis. I don't know much about the SID stuff, but removing the phrase above makes the rest of the paragraph less cohesive.
- Basically, it means that a strong ion or anion, such as lactate, can have a direct effect on the pH, just by changing the ratio of strong ions to strong anions. For example, a large quantity of sodium-lactate, which is basically a strong ion (Na) and a strong anion (lactate), will cause acidosis, because it has a SID of 0, while the human blood has a SID of about 30. Note that this means that even a base like lactate (not lactic acid!), and even though sodium lactate is not capable of releasing a proton, can cause acidosis!
That whole paragraph is wrong, and should be rewritten Terrapin2001 18:02, 2 January 2007 (UTC)
Wrong
"Without lactate, increased concentrations of pyruvate would inhibit glycolysis and greatly reduce ATP production."
Lactate is a product of pyruvate oxidation. But sentence seems to say that lack of lactate causes increase in concentration of pyruvate. What really happens is that lack of lactate and increased concentration of pyruvate can be result of, say, impaired function of lactate dehydrogenase.
Wrong?
If you take the sentence out of the context of the paragraph, then yes it will sound wrong. The paragraph is talking about increases in lactate production, which occurs when pyruvate dehydrogenase doesn't process the pyruvate quick enough (impaired LDH function would be a separate issue). Thus the need for pyr-->la since without it pyruvate would build up and and inhbit glycolysis under conditions where PDH isn't working quick enough. I have edited it nonetheless to make this clearer.
Cosmetic Usage
Shouldnt there be a section discussing the role of lactic acid in skin care? I'll add that in the mean time and discuss if you dont think that belongs there. --Chicbicyclist 14:34, 28 January 2006 (UTC)
ATP factiod highly unlikely; Lactic Acid produced, disassociates
One paragraph states that: "The acidosis that is associated with increases in lactate concentration during heavy exercise arises from a completely separate reaction."
The body produces Lactic acid during anaerobic exercise, which then disassociates into Lactate and free H+ ions, which acidify the blood. Only once the lactate and extra H+ ions pass through the heart and then through the liver are they converted into glucose; this takes some time, yet the article suggests this process happens instantaneously. Also, ATP hydrolysis occurs EVERYWERE in the body ALL THE TIME so a little increase in hydrolysis in the muscles cant be expected to highly acidify the body. Can someone help fix these errors? Or at least explain in a little more detail with a little more clarity.
The article's very own reference [1] correctly identifies Lactic acid as the acidifying culprit. It should also be noted that the extra H+ ions released by Lactic acid cause O2 and haemoglobin to disassociate more readily, aiding in oxygen delivery to the active muscles. 138.16.19.127 00:32, 11 April 2006 (UTC)
Lactic acid isn't produced
1) I'll get rid of that link you've quoted as it is misleading and partially incorrect.
2) The body *doesn't* produce lactic acid during exercise, if you look at the last external link you'll see a thorough discussion on why. Briefly, as it is mentioned in the article, the aren't enough hydrogens present at the end of glycolysis to produce lactic acid at physiological pH. What you produce is lactate since the pKa's of the molecules in glycolysis favour a dissociated state (right from the start) so it is not possible to produce lactic acid as there isn't enough hydrogens i.e. the end of glycolysis produces pyruvate, not pyruvic acid. The old "lactic acid" theory is an unfortunate bit of dogma that just wont die.
3) Lactate doesn't always go to the liver to undergo gluconeogenesis. Some of it diffuses into adjacent muscle fibres to be converted back to pyruvate and then proceeds through the Krebs cycle.
4) The ATP hydrolysis during exercise isn't a *little* bit, it is quite substantial. The reason why we don't become acidotic all the time is because we have buffering systems (namely HCO3) to prevent this. But during heavy exercise, ATP hydrolysis happens at a high rate which essentially depletes the HCO3 and then results in an acidotic state.
5) The section is not specifically referring to acidification of the blood, but just in general as the muscle tissue will also become more acidic.
6) I will review the section and attempt to make this clearer
Lactic acid is produced
Well, it depends on how you look at it. If you look at the reaction from glucose to lactate, the net result is 2 lactate, 2H+ and 2ATP which gives a lower pH. I admit that the acid is dissociated from the very beginning but it's not incorrect to say that the body produces lactic acid, just a bit sloppy. The reason for the lactate buildup is the lack of oxygen needed for the citric acid cycle and the oxidative decarboxylation where the H+ ions are reduced to water. --Eribro 22:27, 3 May 2006 (UTC)
- The reaction from glucose to lactate actually consumes hydrogen. I think you're mixing up the glycolysis and lactate reactions.
- Glucose + 2ADP + 2Pi + 2NAD+ -->2 pyruvate + 2 ATP + 2 NADH + 2H+ + 2H2O
- 2Pyruvate + 2NADH + 2H+ -->2lactate + 2NAD+
- Net is therefore
- Glucose + 2ADP + 2Pi -->2 ATP + 2H2O + 2 lactate
- While lack of oxygen can cause an increase in lactate concentrations, it's not the defining factor per se, it has more to do with the pyruvate dehydrogenase reaction. About 50% of glucose goes to lactate at rest (when we certainly aren't oxygen deprived), but it gets converted back to pyruvate so we don't get any increase in concentration. A minor detail, but I thought you might be interested. Rerun 04:08, 2 June 2006 (UTC)
- yes lactic acid is produced in the body. when parts of your body besides the brain are deprived of oxygen, they obtain energy with a different process. this process is not as effective but it still gets the job done however lactic acid is a by-product from the process.
- The conversion of glucose + ADP + P<sub<i to lactate + ATP is only proton-neutral until the ATP is hydrolyzed, which will happen virtually instantly during heavy exercise. Once the ATP that was formed during glycolysis is consumed, the overall reaction is simply
- glucose → 2H+ + 2 lactate-.
- Most of the protons are neutralized by intracellular buffers (phosphate and proteins), but not all. Therefore, as the muscle is exercised, its pH drops. Quoting from "Biochemistry", 3rd edition, by Reginald Garrett & Charles Grisham, pp 888-889:
- "[Muscle fatigue] is not the result of exhaustion of the glycogen reserves, nor is it a consequence of lactate accumulation in the muscle. Instead, it is caused by a decline in intramuscular pH as protons are generated during glycolysis. (The overall conversion of glucose to 2 lactate in glycolysis is accompanied by the release of 2 H+.) The pH may fall as low as 6.4. It is likely that the decline in PFK activity [PFK = phosphofructokinase, one of the regulatory enzymes in glycolysis] at low pH leads to a lowered flux of hexose through glycolysis and inadequate ATP levels, causing a feeling of fatigue."Topojim (talk) 18:43, 30 July 2009 (UTC)
- yes lactic acid is produced in the body. when parts of your body besides the brain are deprived of oxygen, they obtain energy with a different process. this process is not as effective but it still gets the job done however lactic acid is a by-product from the process.
Vandalism
This page has been vandalized twice by the IP address 71.141.251.75 with senseless bashing of "emo" and "hardcore" music (probably just umbrella terms for music he/she doesn't like). I recommend this IP be blocked immediately.--Terminus-Est 03:48, 22 September 2006 (UTC)
- Blockings are done at WP:AIV. Richard001 07:50, 21 August 2007 (UTC)
Clearance and Use as Energy
I was thinking that there should be some statement on the clearance of lactate into the blood, particularly in respect to its measurement in short-duration, high-intensity exercise. Also, the importance of lactate as an energy source, esp. for the heart. Its metabolism should be discussed, as the Cori Cycle link is woefully inadequate and seems to be dated. —The preceding unsigned comment was added by 207.81.125.94 (talk • contribs) 12:28, 3 December 2006.
Industrial uses
The article has become highly anthropocentric. A good starting point for researching other applications of LA is at http://www.lactic.com/ --Zymatik 20:02, 19 December 2006 (UTC)
pKa
What about its Ka value ? I need it ASAP!
pKa value for lactic acid is 3.86. —Preceding unsigned comment added by 74.184.249.4 (talk) 06:35, 21 January 2010 (UTC)
L and D forms
I was wondering if anyone could tell me, why some bacteria produce both the L and the D form of Lactate?
This is because the D combine with the H particle which in turn is m producing l.
That can't be right, the difference between the L and D form is that they are chiral. So it wouldn't make sence if the L form is produced from adding an ekstra H particle to the D form. The question is, If I werent to clear on it before, Why do some bakteria, when they ferment Lactose to lactate, produce both L and the D form of it? —Preceding unsigned comment added by 195.24.10.179 (talk) 07:37, 15 October 2007 (UTC)
How long ?
Why can't Lactic acid be the main source of energy for the body. I mean it does most of the work...get rid of ATP-PC and aerobic. Woot for lactic acid ! —Preceding unsigned comment added by 124.187.70.5 (talk) 06:10, 15 October 2007 (UTC)
Missing Hydrogen?
Isnt the picture missing a Hydrogen atom? The Carbon only has 3 bonds. —Preceding unsigned comment added by 89.243.227.109 (talk) 01:10, 16 December 2007 (UTC)
Carbon-hydrogen bonds are not typically shown on molecular structure diagrams. By convention, it is assumed that any missing carbon bonds are hydrogen. Sorry if that sounds a tad confusing...for example, the structural drawing of ethane (C2H6) would be a straight line representing the carbon-carbon bond. The remaining 6 carbon-hydrogen bonds are not shown.
Zinc lactate
I noticed zinc lactate listed as a mouthwash ingredient. That page doesn't exist and lactate disambiguates to this page. Would anyone care to start the page zinc lactate? —BenFrantzDale 03:45, 8 March 2008 (UTC)
WikiProject Food and drink Tagging
This article talk page was automatically added with {{WikiProject Food and drink}} banner as it falls under Category:Food or one of its subcategories. If you find this addition an error, Kindly undo the changes and update the inappropriate categories if needed. The bot was instructed to tagg these articles upon consenus from WikiProject Food and drink. You can find the related request for tagging here . Maximum and carefull attention was done to avoid any wrongly tagging any categories , but mistakes may happen... If you have concerns , please inform on the project talk page -- TinucherianBot (talk) 17:41, 3 July 2008 (UTC)
Sprinting
Sprinting isnt really a good example for prduction of lactate. It really depends on how long you are sprinting for because in a 100m sprint your really only using your stored ATP-PC. Wikistiki69 22:25, November 4, 2008
Lactic acid main source of energy
who ever said that in the How Long? bit you are a fool and i hope you were joking and if you wernt joking I'll happily explain why to you. Wikistiki69 22:25, November 4, 2008
Availability at health food stores
Is lactic acid ever available in shaker form, like salt and pepper shakers? Or, if it is hygroscopic, and sucks water out of the air, is it ever available in health food stores, say, mixed with honey? (I wanted to marinade some cuts of beef.) My local grocery store doesn't carry it. While anybody can get it in buttermilk, trying to get a form that has been isolated from milk, appears to be just about impossible. 198.177.27.14 (talk) 07:06, 7 February 2009 (UTC)
Lactic acid very toxic to the brain
thought this might be worth mentioning. lactic acid is very toxic to the brain but does not cause much of a problem for the rest of the body. source- the 'book It's Not a Tumor' by Roberty Wiedemeyer, M.D. page 11 ISBN 0-9647407-9-6
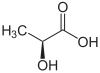
The line drawing of lactic acid is misleading
The line drawing in the infobox illustrates the S isomer. However, an image for a generic lactic acid seems to be intended instead. A different image is needed--Chibibrain (talk) 22:26, 17 November 2009 (UTC)
Agreed. I went to this talk page to see if this had been addressed before. Removing the image would be preferred by me to keeping them the same. The IUPAC name placed directly below the image is incorrect as to the image, and this is harmful and unnecessary.--Δζ (talk) 06:53, 29 May 2010 (UTC)
A simple question
Will eating milk products in any way affect lactate blood levels? --CopperKettle 16:05, 1 February 2010 (UTC)
- Can one manipulate the diet somehow to minimize the lactate levels? --CopperKettle 16:08, 1 February 2010 (UTC)
Barring unforeseen complexity beyond what is covered in basic biochemistry, the answer is a simplistic no. Body regulation should quickly turn any excess lactate back to pyruvate. If one were to drink extreme quantities of lactate (beyond that seen in milk products), perhaps one could saturate the pathway, but I in no way recommend attempting this.
The topic and mosquito
--58.38.45.18 (talk) 08:02, 7 February 2010 (UTC)
--222.64.29.138 (talk) 08:33, 7 February 2010 (UTC)
--222.64.29.138 (talk) 08:34, 7 February 2010 (UTC)
One coin, two sides....
- Allergy - http://scholar.google.com/scholar?hl=en&q=allintitle%3A+lactic+acid+allergy&btnG=Search&as_sdt=2000&as_ylo=&as_vis=0
--222.64.29.138 (talk) 08:38, 7 February 2010 (UTC)
Two questions
Is the 3D picture of the molecule in the upper right corner the D form or the L form?
Lactic acid is often mentioned as an additive on food labels, and I had always assumed it was vegan, being synthetic. Is this correct? If it is synthetic, wouldn't it be a D/L mix? Is the D form non-toxic?
David Olivier (talk) 23:14, 27 February 2010 (UTC)
How are you defining "vegan" and "non-toxic"? I've heard a bunch of conflicting and confusing usages, so can't really answer untill I understand the question. The picture is the S enantiomer, which is stupid in my opinion as it isn't stated as such. Synthesizing lactic acid does not restrict you to any particular stereoisomer ratios, so the composition of synthesized lactic acid can't be deduced by this fact alone.--Δζ (talk) 06:59, 29 May 2010 (UTC)
Lactic Acid used for cleaning ultra filter skid
Can Lactic Acid used for cleaning ultra filter skid used in laquer plating?
Ultra filter smokey but emasculated acid not now.
Chiral conflict?
It seems that the image depicting ethyl lactate is showing the wrong chirality compared to this. Has someone just flipped around the lactic acid without thinking when they made the image of the ester? Which one is right? I am pretty sure, but I would prefer a second editor's opinion. Thanks in advance. --Ephemeronium (talk) 23:05, 24 October 2010 (UTC)
- Okay, they are both right. I've just realized the 'L' means different things with respect to each one. L-Lactic acid is the (+) isomer, whilst L-ethyl lactate is the (-) isomer. In other words they are L-(+)-lactic acid and L-(-)-ethyl lactate. --Ephemeronium (talk) 10:58, 6 November 2010 (UTC)
Mis-tag under Wiki food and drink?
It seems that this, being the biochemical page of Lactate, should not be under food and drink. I believe the page was split to address this issue?
What is a "registered anti-bacterial agent"?
"It is a good descaler, soap-scum remover, and a registered anti-bacterial agent."
What does that mean? With whom is it registered? 178.0.47.202 (talk) 19:08, 17 April 2011 (UTC)
Doubts about objectiveness on article about H+ and conferences
I applaud full descriptions, and think that this question should be reflected on wikipedia (as I use this as a basic source of scientific facts), but am not positive the paragraph looking at le chatlier's principle and strong anions is the scientific consensus, as it leads itself to imply.
- Do you have any recent articles (or conference abstracts, in a pinch) contradicting it? I'm a bit out of my depth on the chemistry side of this page. Mokele (talk) 16:57, 16 March 2011 (UTC)
- Following the links in that paragraph takes us to the very helpful "Articles citing this article" section of the American Journal of Physiology. There have been repeated articles back and forth since (and even before) the Roberts article cited in the Wikipedia entry, implying controversy. A particularly pithy excerpt follows, making it explicit:
- "Lindinger et al. are convinced that SID (essentially [Na+ + K+ – Cl– – La–]), in which [La–] is only a part, determines [H+] in body fluids to a large extent. This concept is disputed in the physiological community" http://jap.physiology.org/content/105/1/358.full Grothmag (talk) 22:11, 7 June 2011 (UTC)
New blood plasma concentration
Hi, I just found a new concentration for normal concentration of lactate in blood plasma and I just want to know if peole agree with it. I found in the lactic acidosis wikipedia page this article (here the reference below) and they say that the lactate concentration is normally 0.4 to 1.0 mmol/L at rest. Since the page has no reference to prove that the concentration is 1 to 2 mmol/L I propose to change the information with the new one I found. I know it is not very scientific with only one reference but hey! It is a beginning and if other people find other reference which state the normal concentration it can help this article. I just want to say thank you for everybody who work on this article and I hope i have been able improve this article (this is my first time). The article below is free and sory for my poor/bad english.
Luft, FC (February 1, 2001). "Lactic acidosis update for critical care clinicians". Journal of the American Society of Nephrology (American Society of Nephrology) 12 (Suppl. 17): 15–19. PMID 11251027. Retrieved 2008-05-28.
Domgaluf (talk) 00:39, 12 December 2012 (UTC)
References
- ^ Luft, FC (1). "Lactic acidosis update for critical care clinicians". Journal of the American Society of Nephrology. 12 (Suppl. 17): S15 – S19. PMID 11251027.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help)
To cast a doubt upon the pKa shift rationale
Re: "This higher acidity is the consequence of the intramolecular hydrogen bridge between the α-hydroxyl and the carboxylate group, making the latter less capable of strongly attracting its proton."
Steric factors would not look too favorably upon the presumably forming single molecule structure, lactide takes 2 molecules for a reason. It is the intermolecular hydrogen bonds that are likely to utilize the hydrogen of the α-hydroxy group. Do note the way boiling point shoots up: from 144 °C of propanoic acid to some 260 °C in lactic acid, a shift quite not explainable either with intra-molecular interaction or by relatively higher weight of an oxygen atom. It's a definite sign of increased intermolecular bonds strength.
As the pKa goes, it's due to inductive effect of hydroxyl, weakened to a degree by an extra carbon-carbon bond in the chain. It effectively decreases the absolute value of the negative charge on carboxylate group oxygens, stabilizing the anion compared to acetic acid with its weak positive inductive effect towards the carboxylic group. Legate of Skai (talk) 20:14, 10 October 2013 (UTC)
arterial vs venous sampling
My understanding is that it's generally acceptable to use venous samples for lactate sampling.
Lavery RF, Livingston DH, Tortella BJ, Sambol JT, Slomovitz BM, Siegel JH. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg 2000;190:656-64. Younger JG, Falk JL, Rothrock SG. Relationship between arterial and peripheral venous lactate levels. Acad Emerg Med 1996;3:730-4. Middleton P, Kelly AM, Brown J, Robertson M. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J 2006;23:622-4
Thanks to Scott Weingard for references http://emcrit.org/wp-content/uploads/lactate-faq.pdf — Preceding unsigned comment added by 82.44.149.214 (talk) 20:23, 20 October 2015 (UTC)
Health Effects: Dental Caries, Galactose, (and Brain)
Wikipedia article now says: "Lactic acid fermentation is performed by Lactic Acid Bacteria, which convert simple carbohydrates such as glucose, sucrose, or galactose to Lactic acid. These bacteria can also grow in the mouth; the acid they produce is responsible for the tooth decay known as caries". I have some questions about Sour milk, Yogurt or any other milk based drink that is made with Lactid Acid Bacteria:
1) Has it been shown drinking a milk based drink containing Lactic Acid Bacteria causes Dental Caries: enamel caries or dentin caries? Perhaps eating hard cheese, like emmental or edam is safer, altough cheese also has pH below 7?
2) How much of Galactose converts to Lactid Acid in these drinks? Many researches believe Galactose is harmful for health, similar to Fructose in health effects, if one compares them to Glucose.
3) Benefits for Brain? (I suppose it is better idea to just do AnAerobic exercise). http://www.pharmaceutical-journal.com/opinion/blogs/lactic-acid-found-to-have-a-role-as-a-brain-fuel/10038374.blog
ee1518 (talk) 11:17, 15 April 2016 (UTC)
Picture of lactic acid
Lactic acid is solid at room temperature but the picure of it is in a liquid. The picture should be titled Lactic acid solution or have a picture of lactic acid material in its solid form. — Preceding unsigned comment added by 148.177.96.116 (talk) 18:59, 11 May 2016 (UTC)
- If I understood correctly, the pure D and L isomers have melting points above 50 C, but the racemic mixture melts at about 17 C. So that bottle could be the latter. Anyway, a picure of the solid pure forms should be shown too. --Jorge Stolfi (talk) 03:09, 14 April 2017 (UTC)
Vandalism
Only the wikipedia app shows a small writing under the main title of Lactic acid, usually in latin, except it is an obsenity in spanish. It says "Verga Peluda" it means "hairy penis" in English, and I tried to remove it but it does not show those words in the computer version. Please help, and tell me how to access that main term name/ sub name that is only in the wikipedia app version. Thank you. Curlystallion Curlystallion (talk) 23:50, 31 July 2016 (UTC) 7/31/2016
- That's weird, I have no idea how that works. I can't see it on desktop or mobile browser, but within the Android tablet app, I see exactly what you describe. Yet I can't find it in the page source, either on desktop or through the app. HCA (talk) 18:02, 1 August 2016 (UTC)
- Ok, the folks at the WP Help Desk found it and fixed it - it was vandalism on another Wiki, which is used as a data source for the app. HCA (talk) 22:14, 2 August 2016 (UTC)
Melting point
The melting points of both D- and L- isomers were given as identical, as indeed they must be. I have changed the chembox accordingly. The melting point of a racemic mixture must be the same as the melting point of a single isomer. If there is evidence to the contrary in this case a valid citation must be be added to the article. Petergans (talk) 09:37, 3 June 2017 (UTC)
- See pubchem. JSR (talk) 16:47, 3 June 2017 (UTC)
Lactate and Cancer
Why is there no mention of this??? https://scholar.google.com/scholar?q=lactate+and+cancer&hl=en&as_sdt=0&as_vis=1&oi=scholart&sa=X&ved=0ahUKEwihiaGus9_WAhUJ6oMKHYlNByQQgQMIJjAA — Preceding unsigned comment added by 2605:6000:101F:C07F:C547:3487:7A61:6325 (talk) 21:00, 7 October 2017 (UTC)
What is the main point? What makes you think that is important? That looks like a random Google search.
--ee1518 (talk) 22:51, 11 February 2019 (UTC)
Outdated link
The forty-eighth source, the one about using lactic acid and salt of hartshorn as mosquito lure is outdated and leads to a 404 page. FendSyllin (talk) 17:19, 23 December 2018 (UTC)
- If there is a link to archived page of that link available then you can add it. A link should not be removed only because we can't read it anymore. Shashank5988 (talk) 18:08, 23 December 2018 (UTC)
Solubility
I am confused by the article 2nd sentence: "Solubility is so high that 1 part of lactic acid can dissolve 12 parts of water." Water is the solvent, not the solute, right? Shouldn't this be "Solubility is so high that 1 part of lactic acid can be dissolved in 12 parts of water"? Unfortunately almost all web pages about lactic acid solubility seem to quote this confusing WP sentence. I'm not an expert so someone else will have to fix or clarify this. 173.76.107.169 (talk) 19:12, 28 July 2019 (UTC)