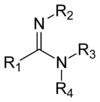
Amidine
Amidines are a class of oxoacid derivatives.
The oxoacid from which an amidine is derived must be of the form RnE(=O)OH, where R is a substituent. The −OH group is replaced by an −NH2 group and the =O group is replaced by =NR, giving amidines the general structure RnE(=NR)NR2.[1][2][3]
Carboxamidines

When the parent oxoacid is a carboxylic acid, the resulting amidine is a carboxamidine or carboximidamide (IUPAC name), and has the following general structure:
Carboxamidines are frequently referred to simply as amidines, as they are the most commonly encountered type of amidine in organic chemistry. The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines include:
The most common way to make primary amidines is by the Pinner reaction. Reaction of the nitrile with acidic alcohol gives an iminoether; treatment of this with ammonia then completes the conversion to the amidine.
Properties
Amidines are much more basic than amides and are among the strongest uncharged/unionized[4] bases.[5]
Protonation occurs onto the sp2 hybridized nitrogen. This occurs because the positive charge can be delocalized onto both nitrogen atoms. The resulting cationic species is known as a amidinium ion[6] and possesses identical C-N bond lengths.

Derivatives
Formamidinium cations

A notable subclass of amidinium ions are the formamidinium cations; which can be represented by the chemical formula [R
2N−CH=NR
2]+
. Deprotonation of these gives stable carbenes which can be represented by the chemical formula R
2N−C:−NR
2.[7]
Amidinate salts
An amidinate salt has the general structure M+[RNRCNR]− and can be accessed by reaction of a carbodiimide with an organometallic compound such as methyl lithium.[8] They are used widely as ligands in organometallic complexes.
See also
- Guanidines — a similar group of compounds where the central Carbon is bonded to three Nitrogens.
- Imidazolines contain a cyclic amidine.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amidines". doi:10.1351/goldbook.A00267
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carboxamidines". doi:10.1351/goldbook.C00851
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfinamidines". doi:10.1351/goldbook.S06107
- ^ Roche VF. Improving Pharmacy Students’ Understanding and Long-term Retention of Acid-Base Chemistry. American Journal of Pharmaceutical Education. 2007;71(6):122.
- ^ Clayden; Greeves; Warren (2001). Organic chemistry. Oxford university press. p. 202. ISBN 978-0-19-850346-0.
- ^ Schrader, Thomas; Hamilton, Andrew D., eds. (2005). Functional synthetic receptors. Wiley-VCH. p. 132. ISBN 3-527-30655-2.
- ^ Alder, Roger W.; Blake, Michael E.; Bufali, Simone; Butts, Craig P.; Orpen, A. Guy; Schütz, Jan; Williams, Stuart J. (2001). "Preparation of tetraalkylformamidinium salts and related species as precursors to stable carbenes". Journal of the Chemical Society, Perkin Transactions 1 (14): 1586–1593. doi:10.1039/B104110J.
- ^ Ulrich, Henri (2007). Chemistry and technology of carbodiimides. Chichester, England: John Wiley & Sons. ISBN 9780470065105.