Pyrosilicic acid
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Trihydroxysilyl trihydrogen orthosilicate
| |
| Other names
disilicic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 26937 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6O7Si2 | |
| Molar mass | 174.211 g·mol−1 |
| Conjugate base | Pyrosilicate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
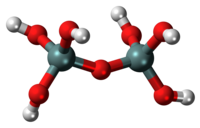
Pyrosilicic acid is the chemical compound with formula (HO)
3Si-O-Si(OH)
3. It was synthesized, using non-aqueous solutions, in 2017.[1] It may be present in sea water and other natural waters at very low concentration.[2][3] Its low solubility in water is due to the preferential formation of its insoluble anhydride, silica, SiO2.
- H6Si2O7 (aq) → 2SiO2↓ + 3H2O
Salts of pyrosilicic acid, such as sodium pyrosilicate are found in nature, in sorosilicates.
References
- ^ Igarashi, Masayasu; Matsumoto, Tomohiro; Yagahashi, Fujio; Yamashita, Hiroshi; Ohhara, Takashi; Hanashima, Takayasu; Nakao, Akiko; Moyosh, Taketo; Sato, Kazuhiko; Shimada, Shigeru (2017). "Non-aqueous selective synthesis of orthosilicic acid and its oligomers". Nature Communications. 8 (1): 140. doi:10.1038/s41467-017-00168-5. PMC 5529440. PMID 28747652.
- ^ Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". Journal of Physical Chemistry, volume 60, issue 7, pages 1007–1008. doi:10.1021/j150541a046
- ^ M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". Journal of Physical Chemistry, volume 59, issue 6, pages 532–541. doi:10.1021/j150528a013
