Silver oxide battery
| Specific energy | 130 Wh/kg |
|---|---|
| Energy density | 240 Wh/L |
| Specific power | High |
| Charge/discharge efficiency | N/A |
| Energy/consumer-price | Low |
| Self-discharge rate | Negligible |
| Time durability | High |
| Cycle durability | N/A |
A Silver-oxide battery, also called a silver-zinc battery, is a primary cell with an open circuit voltage of 1.6 volts. Silver-oxide batteries have a long life and very high energy/weight ratio, but prohibitive cost for most applications due to high price of silver. They are available in either very small sizes as button cells where the amount of silver used is small and not a significant contributor to the overall product costs, or in large custom design batteries where the superior performance characteristics of the silver-oxide chemistry outweigh any cost considerations. The large cells found some applications in military, eg. in the Mark 37 torpedoes or on Alfa class submarines.
A silver oxide battery is a small-sized primary battery using zinc as the negative electrode (anode), silver oxide as the positive electrode (cathode) plus an alkaline electrolyte, usually sodium (NaOH) or potassium hydroxide (KOH). The chemical reaction that takes place inside the battery is the following:
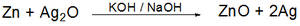
Zinc is the activator in the negative electrode and corrodes in alkaline solution. When this happens, it becomes difficult to maintain the capacity of the unused battery. The zinc corrosion causes electrolysis in the electrolyte, resulting in the production of hydrogen gas, a rise of inner pressure and expansion of the cell. Mercury has been used in the past to suppress the corrosion, despite its harmful effects on the environment.
Compared to other batteries, a silver-oxide battery has a higher closed circuit voltage than a mercury battery, and a flatter discharge curve than a standard alkaline battery.
