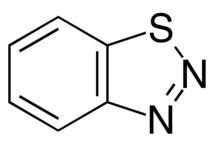
1,2,3-Benzothiadiazole
| |
| Names | |
|---|---|
| IUPAC name
1,2,3-Benzothiadiazole
| |
| Systematic IUPAC name
1,2,3-Benzothiadiazole | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | benzo-1,2,3-thiadiazole |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4N2S | |
| Molar mass | 136.17 g·mol−1 |
| Melting point | 36–37 °C (97–99 °F; 309–310 K) |
| Boiling point | 220.5 °C (428.9 °F; 493.6 K) |
| Related compounds | |
Related compounds
|
2,1,3-Benzothiadiazole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,2,3-Benzothiadiazole is a bicyclic aromatic chemical composed of a benzene ring that is fused to a 1,2,3-thiadiazole.
Preparation
1,2,3-Benzothiadiazole is readily prepared by the diazotisation reaction of 2-aminothiophenol or its disulfide with sodium nitrite, as originally reported in 1887[1] and reviewed in several subsequent publications.[2][3][4][5]
By the Herz reaction anilines can be converted to benzothiadiazole. The method is attractive since less elaborate precursors (merely anilines) are required. Upon treatment with disulfur dichloride, the anilines give the intermediate 1,3,2-benzothiazathiolium salt, which is diazotised to complete the formation of a 1,2,3-benzothiadiazole. The parent system cannot be made this way, since the use of aniline in this reaction leads to formation of the 6-chloro derivative.[6]
Structure and bonding
The molecule is planar.[7]
The extent of the aromaticity of the compound was examined by comparison with naphthalene, which allowed the conclusion that it behaved as a 10-electron system in which the sulfur atom contributes a lone pair to the pi-system.[2]
Reactions
1,2,3-benzothiadiazole is much less nucleophilic than naphthalene. Nitration is slow.[8] For that reason, many of its simple derivatives have been made either from substituted 2-aminothiophenols.[6]
1,2,3-benzothiadiazole is a very weak base and alkylation reactions give exclusively the 3-amino quaternary salt.[9]
Applications
1,2,3-benzothiadiazole has been claimed to synergise insecticides including dicrotophos[10] but has not been commercialised for that application. The only derivative to have found significant use is the fungicide acibenzolar-S-methyl.
References
- ^ Jacobson, J. (1887). "Zur Kenntniss der orthoamidirten aromatischen Mercaptane". Berichte der Deutschen Chemischen Gesellschaft. 20: 1895–1903. doi:10.1002/cber.188702001423.
- ^ a b Hodgson, H.H.; Dodoson, D.P. (1964). "A Review of the Chemistry of the Arylthiadiazoles or Internal Diazo–Sulphides". Journal of the Society of Dyers and Colourists. 64 (2): 65–71. doi:10.1111/j.1478-4408.1948.tb02498.x.
- ^ Thomas, E.W. (1984). "1,2,3-Thiadiazoles and their Benzo Derivatives". Comprehensive Heterocyclic Chemistry. pp. 447–462. doi:10.1016/B978-008096519-2.00092-8. ISBN 9780080965192.
- ^ Storr, R. C.; Gilchrist, T. L., eds. (2004). "Product Class 9: 1,2,3-Thiadiazoles". Science of Synthesis. Vol. 13: Category 2, Hetarenes and Related Ring Systems. doi:10.1055/sos-SD-013-00386. ISBN 9783131122810.
- ^ "1,2,3-Benzothiadiazole". Houben-Weyl Methods of Organic Chemistry Vol. E 8d, 4th Edition Supplement: Hetarenes III (Five-Membered Rings with Two and More Heteroatoms in the Ring System) - Part 4. 14 May 2014. pp. 93–104. ISBN 9783131812445.
- ^ a b Kirby, P.; Soloway, S. B.; Davies, J. H.; Webb, Shirley B. (1970). "1,2,3-Benzothiadiazoles. Part I. A simplified synthesis of 1,2,3-benzothiadiazoles". Journal of the Chemical Society C: Organic (16): 2250. doi:10.1039/J39700002250.
- ^ . doi:10.1016/0022-328X(91)86290-7.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Ward, E. R.; Heard, D. D. (1963). "1,2,3-Benzothiadiazole. Part II. Electrophilic substitution in 4- and 6-amino-1,2,3-benzothiadiazoles". Journal of the Chemical Society (Resumed): 4794–4803. doi:10.1039/JR9630004794.
- ^ Jaffari, G. A.; Nunn, A. J.; Ralph, J. T. (1970). "1,2,3-Benzothiadiazole. Part VI. Investigations on the quaternisation of 1,2,3-benzothiadiazole and 1,2,3-benzoselenadiazole". Journal of the Chemical Society C: Organic (15): 2060. doi:10.1039/J39700002060.
- ^ Felton, John C.; Jenner, Donald W.; Kirby, Peter. (1970). "Benzothiadiazoles, a novel group of insecticide synergists". Journal of Agricultural and Food Chemistry. 18 (4): 671–673. doi:10.1021/jf60170a011.

