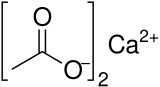
Calcium acetate
| |
 Calcium acetate crystals
| |
| Names | |
|---|---|
| Preferred IUPAC name
Calcium diacetate[1] | |
| Other names
Acetate of lime
Calcium ethanoate | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | Ca(OAc)2 |
| 3692527 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.492 |
| EC Number |
|
| E number | E263 (preservatives) |
| 22320 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6CaO4 | |
| Molar mass | 158.166 g/mol (anhydrous) 176.181 g/mol (monohydrate) |
| Appearance | White solid hygroscopic |
| Odor | slight acetic acid odor |
| Density | 1.509 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K)[2] decomposition to CaCO3 + acetone |
| 37.4 g/100 mL (0 °C) 34.7 g/100 mL (20 °C) 29.7 g/100 mL (100 °C) | |
| Solubility | slightly soluble in methanol, hydrazine insoluble in acetone, ethanol and benzene |
| Acidity (pKa) | ca. 0.7 |
| -70.7·10−6 cm3/mol | |
Refractive index (nD)
|
1.55 |
| Pharmacology | |
| V03AE07 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| 680 to 730 °C (1,256 to 1,346 °F; 953 to 1,003 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4280 mg/kg (oral, rat) |
| Related compounds | |
Other cations
|
Magnesium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form.
Production
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as limestone or marble) or hydrated lime in vinegar:
- CaCO3(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + H2O(l) + CO2(g)
- Ca(OH)2(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + 2H2O(l)
Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Uses
- In kidney disease, blood levels of phosphate may rise (called hyperphosphatemia) leading to bone problems. Calcium acetate binds phosphate in the diet to lower blood phosphate levels.[3]
- Calcium acetate is used as a food additive, as a stabilizer, buffer and sequestrant, mainly in candy products under the number E263.
- Tofu is traditionally obtained by coagulating soy milk with calcium sulfate. Calcium acetate has been found to be a better alternative; being soluble, it requires less skill and a smaller amount.[4]
- Because it is inexpensive, calcium acetate was once a common starting material for the synthesis of acetone before the development of the cumene process:[5][6]
- Ca(CH3COO)2 → CaCO3(s) + (CH3)2CO
- A saturated solution of calcium acetate in alcohol forms a semisolid, flammable gel that is much like "canned heat" products such as Sterno.[7] Chemistry teachers often prepare "California Snowballs", a mixture of calcium acetate solution and ethanol.[8] The resulting gel is whitish in color, and can be formed to resemble a snowball.
Natural occurrence
Pure calcium acetate is yet unknown among minerals. Calclacite—calcium acetate chloride pentahydrate—is listed as a known mineral,[9] but its genesis is likely anthropogenic (human-generated, as opposed to naturally occurring).[10][failed verification]
References
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 801. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Dale L. Perry (May 19, 2011). Handbook of Inorganic Compounds (Second ed.). Taylor & Francis. p. 84. ISBN 978-1-4398-1461-1.
- ^ "Calcium Acetate". Mayo Clinic. Retrieved 19 November 2019.
- ^ Lu, J. Y.; Carter, Eloise; Chung, R. A. (1980). "Use of Calcium Salts for Soybean Curd Preparation". Journal of Food Science. 45: 32–34. doi:10.1111/j.1365-2621.1980.tb03864.x.
- ^ Leo Frank Goodwin; Edward Tyghe Sterne (1920). "Losses Incurred in the Preparation of Acetone by the Distillation of Acetate of Lime". Industrial & Engineering Chemistry. 12 (3): 240–243. doi:10.1021/ie50123a012.
- ^ E. G. R. Ardagh; A. D. Barbour; G. E. McClellan; E. W. McBride (1924). "Distillation of Acetate of Lime". Industrial & Engineering Chemistry. 16 (11): 1133–1139. doi:10.1021/ie50179a013.
- ^ ""Canned Heat" at Journal of Chemical Education "Chemistry comes alive!"". Archived from the original on 2008-10-03. Retrieved 2008-03-26.
- ^ Chemistry Teaching Resources
- ^ "Calclacite".
- ^ RRUFF (13 Jan 2024). "Database of Mineral Properties". International Mineralogical Association. Calclacite (dropdown option at left).

