Methylmalonic acidemias
| Methylmalonic acidemias | |
|---|---|
| Other names | Methylmalonic acidurias, MMAs |
 | |
| Methylmalonic acid | |
| Specialty | Endocrinology |
Methylmalonic acidemias, also called methylmalonic acidurias,[note 1] are a group of inherited metabolic disorders, that prevent the body from properly breaking down proteins and fats.[1] This leads to a buildup of a toxic level of methylmalonic acid in body liquids and tissues. Due to the disturbed branched-chain amino acids (BCAA) metabolism, they are among the classical organic acidemias.[2]
Methylmalonic acidemias have varying diagnoses, treatment requirements and prognoses, which are determined by the specific genetic mutation causing the inherited form of the disorder.[3]
The first symptoms may begin as early as the first day of life or as late as adulthood.[4] Symptoms can range from mild to life-threatening.[1] Some forms can result in death if undiagnosed or left untreated.
Methylmalonic acidemias are found with an equal frequency across ethnic boundaries.[5]
Symptoms and signs
Depending on the affected gene(s) and mutation, the present symptoms can range from mild to life-threatening.
- Acidosis[6]
- Cardiomyopathy[7][8]
- Coma[9]
- Dehydration[10][6][11]
- Developmental delays[10][6][11]
- Dysmorphic features[7][8]
- Encephalopathy, progressive[10]
- Failure to thrive[10][6][11]
- Gastrointestinal disease[7][8]
- Hepatomegaly[6][11]
- Hyperammonemia[6]
- Hyperglycinemia[6]/ Hyperglycinuria[6]
- Hypoglycemia[6]
- Hypotonia[6][11]
- Infections, recurrent [10]
- Ketonemia[6]/ Ketonuria[6]
- Kidney failure[10][11]
- Lethargy[10][6][11]
- Low concentrations of red blood cells, white blood cells and blood platelets[6]
- Memory problems[9]
- Pancreatitis[11]
- Respiratory distress[6]
- Speech delay[9]
- Seizure[10][6]
- Stroke[10]
- Vomiting[10][6][11]
As a rule, methylmalonic acidemias are not apparent at birth as symptoms do not present themselves until proteins are added to the infant's diet.[10] Because of this, symptoms typically manifest anytime within the first year of life.[12] However, there are also forms that only develop symptoms in adulthood.[4]
Cause
Genetic
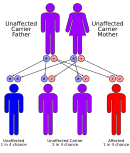
Methylmalonic acidemias have an autosomal recessive inheritance pattern, which means the defective gene is located on an autosome, and two copies of the gene—one from each parent—must be inherited to be affected by the disorder. The parents of a child with an autosomal recessive disorder are carriers of one copy of the defective gene, but are usually not affected by the disorder.[citation needed] The exception is methylmalonic acidemia and homocystinuria, cblX type due to variants in HCFC1 gene, which is inherited in an X-linked recessive manner.[13]
The following are the known genotypes responsible for isolated methylmalonic acidemias:[13]
| Gene | Type | OMIM | Name | Prevalence | Age of onset |
|---|---|---|---|---|---|
| MCEE | 251120 | Methylmalonic acidemia due to methylmalonyl-CoA epimerase deficiency | <1:1,000,000[14] | Childhood, Infancy[14] | |
| MMAA | cblA | 251100 | Methylmalonic acidemia, vitamin B12-responsive, cblA type | <1:1,000,000[15] | Childhood[15] |
| MMAB | cblB | 251110 | Methylmalonic acidemia, vitamin B12-responsive, cblB type | Childhood[16] | |
| MMADHC | cblDv2 | 277410 | Methylmalonic acidemia, cblD type, variant 2 | ||
| MMUT | mut0 | 251000 | Methylmalonic acidemia, vitamin B12-unresponsive, mut0 type | Infancy, Neonatal[17] | |
| mut- | Methylmalonic acidemia, vitamin B12-unresponsive, mut- type | Infancy, Neonatal[18] |
The mut type can further be divided into mut0 and mut- subtypes, with mut0 characterized by a complete lack of methylmalonyl-CoA mutase and more severe symptoms and mut- characterized by a decreased amount of mutase activity.[5]
Furthermore, the following genes are also responsible for methylmalonic acidemias:[13][19]
| Gene | Type | OMIM | Name | Prevalence | Age of onset |
|---|---|---|---|---|---|
| ABCD4 | cblJ | 614857 | Methylmalonic acidemia and homocystinuria, cblJ type | <1:1,000,000[20] | Infancy, Neonatal[20] |
| ACSF3 | 614265 | Combined malonic and methylmalonic aciduria (CMAMMA) | 1:30,000[9] | All ages[21] | |
| ALDH6A1 | 614105 | Methylmalonate semialdehyde dehydrogenase deficiency | <1:1,000,000[22] | Infancy, Neonatal[22] | |
| AMN | 618882 | Imerslund-Grasbeck syndrome 2 | Childhood[23] | ||
| CBLIF | 261000 | Intrinsic factor deficiency | <1:1,000,000[24] | Childhood[24] | |
| CD320 | TcblR | 613646 | Methylmalonic acidemia due to transcobalamin receptor defect | <1:1,000,000[25] | Infancy, Neonatal[25] |
| CUBN | 261100 | Imerslund-Grasbeck syndrome 1 | Childhood[23] | ||
| HCFC1 | cblX | 309541 | Methylmalonic acidemia and homocystinuria, cblX type | <1:1,000,000[26] | Infancy, Neonatal[26] |
| LMBRD1[27] | cblF | 277380 | Methylmalonic acidemia and homocystinuria, cblF type | <1:1,000,000[28] | Childhood[28] |
| MLYCD | 248360 | Malonic aciduria | <1:1,000,000[29] | Childhood[29] | |
| MMACHC, PRDX1 | cblC | 277400 | Methylmalonic acidemia and homocystinuria, cblC type | 1:200,000[30] | All ages[31] |
| MMADHC[32] | cblD | 277410 | Methylmalonic acidemia and homocystinuria, cblD type | <1:1,000,000[33] | All ages[33] |
| SUCLA2 | 612073 | SUCLA2-related mtDNA depletion syndrome, encephalomyopathic form with methylmalonic aciduria | <1:1,000,000[34] | Infancy[34] | |
| SUCLG1 | 245400 | SUCLG1-related mtDNA depletion syndrome, encephalomyopathic form with methylmalonic aciduria | Infancy, Neonatal[35] | ||
| TCN2 | 275350 | Transcobalamin-II deficiency | <1:1,000,000[36] | Infancy, Neonatal[36] | |
| ZBTB11 | 618383 | Autosomal recessive intellectual developmental disorder 69 |
Nutritional
Though not always grouped together with the inherited versions, a severe nutritional vitamin B12 deficiency can also result in syndrome with identical symptoms and treatments as the genetic methylmalonic acidemias.[37] Methylmalonyl-CoA requires vitamin B12 to form succinyl-CoA. When the amount of B12 is insufficient for the conversion of cofactor methylmalonyl-CoA into succinyl-CoA, the buildup of unused methylmalonyl-CoA eventually leads to methylmalonic acidemia. This diagnosis is often used as an indicator of vitamin B12 deficiency in serum.[38]
Pathophysiology

In methylmalonic acidemias, the body is unable to break down properly:
- essential amino acids: methionine, valine, threonine and isoleucine[39]
- propionic acid from intestinal fermentation[39]
- odd-chain fatty acids[39]
- cholesterol side chain[39]
As a result methylmalonic acid builds up in liquids and tissues. Those afflicted with this disorder are either lacking functional copies or adequate levels of one or more of the following enzymes:[6][11][9]
- methylmalonyl-CoA mutase (MUT)
- acyl-CoA synthetase family member 3 (ACSF3)
- methylmalonyl-CoA epimerase (MCEE)
- enzymes involved in adenosylcobalamin synthesis
These are briefly introduced below:
Methylmalonyl-CoA mutase
It is estimated that as many as 60% of isolated methylmalonic acidemia cases are the result of a mutated MMUT gene which encodes the protein methylmalonyl-CoA mutase. This enzyme is responsible for the digestion of potentially toxic derivatives of the breakdown of the above-mentioned amino acids and fats, primarily cholesterol,[11] particularly this enzyme converts methylmalonyl-CoA into succinyl-CoA.[40] Without this enzyme, the body has no means to neutralize or remove methylmalonic acid and related compounds. The action of this enzyme can also be crippled by mutations in the MMAA, MMAB, and MMADHC genes, each of which encodes a protein required for normal functioning of methylmalonyl-CoA mutase.[11]
Acyl-CoA synthetase family member 3
CMAMMA is probably the most common form of methylmalonic acidemia, but is rarely diagnosed due to slippage through routine newborn screening, wide symptom variety and, in some cases, symptoms only appearing in adulthood.[9][41] Mutations of the ACSF3 gene leads to a deficiency of the mitochondrial enzyme acyl-CoA synthetase family member 3, resulting in increased levels of methylmalonic acid and malonic acid.[9] Since the enzyme's task is both the conversion of methylmalonic acid into methylmalonyl-CoA, so that it can be fed into the citric acid cycle, and the conversion of malonic acid into malonyl-CoA, which is the first step in mitochondrial fatty acid synthesis (mtFASII).[42][43] CMAMMA can therefore be defined not only as an organic acidemia but also as a defect of mitochondrial fatty acid synthesis.[43]
Methylmalonyl-CoA epimerase
Mutations in the MCEE gene, which encodes the methylmalonyl-CoA epimerase protein, also referred to as methylmalonyl racemase, will cause a much more mild form of the disorder than the related methylmalonyl-CoA mutase variant. Like the mutase, the epimerase also functions in breaking down the same substances, but to a significantly lesser extent than the mutase does.[11] The phenotypic differences caused by a deficiency of the epimerase as opposed to the mutase are so mild that there is debate within the medical community as to whether or not this genetic deficiency can be considered a disorder or clinical syndrome.[44]
Adenosylcobalamin
Also known as vitamin B12, this form of cobalamin is a required cofactor of methylmalonyl-CoA mutase. Even with a functional version of the enzyme at physiologically normal levels, if B12 cannot be converted to this active form, the mutase will be unable to function.[11]
Diagnosis
Newborn Screening
Due to the severity and rapidity in which this disorder can cause complications when left undiagnosed, screening for methylmalonic acidemia is often included in the newborn screening exam.[10][45] For this purpose, a dried blood spot test for the parameter propionylcarnitine (C3) is carried out at the age of 24–48 hours in order to detect isolated methylmalonic acidemias.[13][46]
Due to normal propionylcarnitine levels and asymptomatic symptoms at the time of testing, the probably most common form of methylmalonic acidemias, CMAMMA, slips through the newborn screening.[9][13] The autosomal recessive intellectual development disorder 69 also has normal propionylcarnitine levels.[13] Methylmalonic acidemia and homocystinuria, cblC type, if mild and with late onset, can also slip through.[47]
Routine & biochemical labs
Typically, the parameter methylmalonic acid is only tested if propionylcarnitine was previously elevated.[48]
Because of the inability to properly break down amino acids completely, the byproduct of protein digestion, the compound methylmalonic acid, is found in a disproportionate concentration in the blood and urine of those afflicted. These abnormal levels are used as the main diagnostic criteria for diagnosing the disorder. This disorder is typically determined through the use of a urine analysis or blood panel.[12] Elevated levels of ammonia, glycine, and ketone bodies may also be present in the blood and urine.[6]
With the inclusion of the parameter malonic acid, CMAMMA can be quickly differentiated from classic methylmalonic acidemia by calculating the ratio of malonic acid to methylmalonic acid, but only with values from the blood plasma and not from the urine.[49] The ratio can then also be used to determine whether it is CMAMMA (MA<MMA) or malonic aciduria (MA>MMA).[49][7][50]
Vitamin B12 responsiveness test
The test is used for further differential diagnosis and to check the effectiveness of treatment with vitamin B12, the latter can prevent unnecessary injections (of vitamin B12) in children.[51] For better comparability and interpretation of patient reports, Fowler et al have developed a protocol for a standardized vitamin B12 responsiveness test (in vivo):[51]
- Metabolically stable and on the same treatment for at least a month. Specify energy and protein intake.
- Stop vitamin B12 at least one month before. If worsening, discontinue and resume administration of vitamin B12.
- For baseline, collect urine from 3 different days. Blood plasma concentrations can also be used if test is sensitive enough.
- Intramuscular injection of 1 mg hydroxocobalamin on 3 consecutive days.
- After injection, collect urine or plasma samples on alternate days for 10 days.
- The urine or plasma samples should be analyzed in the same run by GCMS.
- If the mean urine or plasma concentration of methylmalonic acid decreases by more than 50%, it is vitamin B12 responsive.
Furthermore, vitamin B12 responsiveness can also be tested in vitro.[13][51] It can provide some insights, but it cannot always correctly predict in vivo vitamin B12 responsiveness.[13]
Molecular genetic testing
The final diagnosis is confirmed by molecular genetic testing if biallelic pathogenic variants are found in the affected gene(s). Due to their high sensitivity, easier accessibility and non-invasiveness, molecular genetic tests replace enzyme assays in most cases.[13] There are specific multigene panels for methylmalonic acidemia, but the particular genes tested may vary from laboratory to laboratory and can be customized by the clinician to the individual phenotype.[13][19] The molecular genetic methods used in these panels range from sequence analysis, deletion/duplication analysis and other non-sequencing based tests, but in the vast majority of cases the diagnosis is made by sequence analysis.[13]
Furthermore, molecular genetic tests are necessary to check suspected diagnoses and correct misdiagnoses that may have been caused by misleading symptoms and results of the vitamin B12 responsiveness test.[52]
Other
The presence of methylmalonic acidemia can also be suspected through the use of a CT or MRI scan, however these tests are by no means specific and require clinical and metabolic/correlation.[10]
| Methylmalonic acid levels | Homocysteine levels | Differential diagnosis | Next diagnostics | Vitamin B12 response (in vivo) | Differential diagnosis | Next diagnostics | |
|---|---|---|---|---|---|---|---|
| Methylmalonic acid levels | Homocysteine levels | ||||||
| Very high | Normal | mut0, mut-, cblA, cbIB,
cblDv2 |
Vitamin B12 response (in vivo) | Unresponsive | mut0, mut-, cbIB | Molecular genetic testing, enzyme assay, 14C propionate incorporation, cobalamin complementation studies | |
| Responsive (reduction of >50% or normal levels) | cbIA, cbIB, cbIDv2, mut-? | ||||||
| High | MCEE, TcbIR, SUCLG1/A2, CMAMMA, MMSDH and other | Vitamin B12 response in vivo), enzyme assay, molecular genetic testing | Unresponsive | MCEE, CMAMMA, and other | |||
| Responsive (reduction of >50% or normal levels) | TcblR | ||||||
| High | cblC,cbID, cblF, cblJ, cbIX, TC-II, TcbIR, B12 deficiency syndromes | Vitamin B12 response (in vivo) | High or normal | High or normal | cblC,cbID, cblF, cblJ | ||
| Normal | Normal | TC-II, TcbIR, B12 deficiency syndromes | |||||
| False positive, maternal B12 deficiency | |||||||
Treatment
Dietary
Treatment for all forms of this condition primarily relies on a low-protein diet, and depending on what variant of the disorder the individual suffers from, various dietary supplements. All variants respond to the levo isomer of carnitine as the improper breakdown of the affected substances results in sufferers developing a carnitine deficiency. The carnitine also assists in the removal of acyl-CoA, buildup of which is common in low-protein diets by converting it into acyl-carnitine which can be excreted in urine. Some forms of methylmalonyl acidemia are responsive to cobalamin although cyanocobalamin supplements could prove detrimental to some forms.[53] If the individual proves responsive to both cobalamin and carnitine supplements, then it may be possible for them to ingest substances that include small amounts of the problematic amino acids isoleucine, threonine, methionine, and valine without causing an attack.[10] CblA und cblB versions of methylmalonic acidemia have been found to be cobalamin responsive.[citation needed]
Surgical
A more extreme treatment includes kidney or liver transplant from a donor without the condition. The foreign organs will produce a functional version of the defective enzymes and digest the methylmalonic acid, however all of the disadvantages of organ transplantation are of course applicable in this situation.[10] There is evidence to suggest that the central nervous system may metabolize methylmalonyl-CoA in a system isolated from the rest of the body. If this is the case, transplantation may not reverse the neurological effects of methylmalonic acid previous to the transplant or prevent further damage to the brain by continued build up.[54][40]
mRNA therapeutics
Preclinical proof-of-concept studies in animal models have shown that mRNA therapy is also suitable for rare metabolic diseases, including isolated methylmalonic acidemia.[55][56] In this context, the mut methylmalonic acidemia therapy candidate mRNA-3705 from the biotechnology company Moderna, which is currently in phase 1/2, is worth mentioning.[57]
Small molecular therapeutics
The investigational small molecular therapeutic HST5040 from HemoShear Therapeutics for methylmalonic aciduria and propionic aciduria, which is currently in phase 2, should be mentioned here.[58][59] Taken daily orally or by gastric tube, it is designed to prevent toxic accumulation of propionyl-CoA and methylmalonyl-CoA or their derivatives by shunting CoA away from the propionyl-CoA pathway, leading to normal or near-normal levels of these metabolites and potentially improving metabolic state and energy-producing pathways.[60][59]
Another small molecule therapeutic in development is BBP-671 from BridgeBio Pharma for pantothenate kinase-associated neurodegeneration (PKAN), propionic and methylmalonic acidemia, which is currently in phase 1.[61] By allosterically activating pantothenate kinases, BBP-671 is expected to increase the production of CoA from vitamin B5 and thus normalize metabolic processes.[62]
Prognosis
Though there are not distinct stages of the disease, methylmalonic acidemia is a progressive condition; the symptoms of this disorder are compounded as the concentration of methylmalonic acid increases. If the triggering proteins and fats are not removed from the diet, this buildup can lead to irreparable kidney or liver damage and eventually death.[10]
The prognosis will vary depending on the severity of the condition and the individual's response to treatment. Prognosis is typically better for those with cobalamin-responsive variants and not promising in those suffering from noncobalamin-responsive variants.[40] Milder variants have a higher frequency of appearance in the population than the more severe ones.[12] Even with dietary modification and continued medical care, it may not be possible to prevent neurological damage in those with a nonresponsive acidemia.[40] Without proper treatment or diagnosis, it is not uncommon for the first acidemic attack to be fatal.[10]
Despite these challenges, since it was first identified in 1967, treatment and understanding of the condition has improved to the point where it is not unheard of for even those with unresponsive forms of methylmalonic acidemia to be able to reach adulthood and even carry and deliver children safely.[54]
Research
Nosologic history
The first methylmalonic acidemia was characterized by Oberholzer et al. in 1967.[63][54]
Neurologic effects
That methylmalonic acid can have disastrous effects on the nervous system has been long reported; however, the mechanism by which this occurs has never been determined. Published in 2015, research performed on the effects of methylmalonic acid on neurons isolated from fetal rats in an in vitro setting using a control group of neurons treated with an alternate acid of similar pH.[64] These tests have suggested that methylmalonic acid causes decreases in cellular size and increase in the rate of cellular apoptosis in a concentration dependent manner with more extreme effects being seen at higher concentrations.[64] Furthermore, micro-array analysis of these treated neurons have also suggested that on an epigenetic-level methylmalonic acid alters the transcription rate of 564 genes, notably including those involved in the apoptosis, p53, and MAPK signaling pathways.[64]
Mitochondrial dysfunction
As the conversion of methylmalonyl-CoA to succinyl-CoA takes place inside the mitochondria, mitochondrial dysfunction as a result of diminished electron transport chain function has long been suspected as a feature in methylmalonic acidemias. Recent[when?] research has found that in rat models mitochondria of rats affected by the disorder grow to unusual size, dubbed megamitochondria. These megamitochondria also appear to have deformed internal structures and a loss in electron richness in their matrix. These megamitochondria also showed signs of decreased respiratory chain function, particularly in respiratory complex IV which only functioned at about 50% efficiency. Similar changes were identified in the mitochondria of a liver sample removed during transplant from a 5-year-old boy suffering from methylmalonic acidemia mut type.[65]
Benign mut phenotype
Case studies in several patients presenting nonresponsive mut0 methylmalonic acidemia with a specific mutation designated p.P86L have suggested the possibility of further subdivision in mut type methylmalonic acidemia might exist.[66] Though currently unclear if this is due to the specific mutation or early detection and treatment, despite complete nonresponse to cobalamin supplements, these individuals appeared to develop a largely benign and near completely asymptomatic version of methylmalonic acidemia.[66] Despite consistently showing elevated methylmalonic acid in the blood and urine, these individuals appeared for the large part developmentally normal.[66]
Notable cases
- Ryan Stallings, a St. Louis infant, was mistakenly diagnosed with ethylene glycol poisoning instead of methylmalonic acidemia in 1989, leading to a wrongful murder conviction and life sentence for his mother, Patricia Stallings.[54]
See also
Notes
- ^ The names methylmalonic acidemia and methylmalonic aciduria, which are also sometimes written as solid compounds (methylmalonicacidemia and methylmalonicaciduria), use the suffixes -emia and -uria and literally mean "[excess] methylmalonic acid in the blood" and "[excess] methylmalonic acid in the urine", respectively; they are used to label both the fluid analysis findings and the disease entity that causes them.
References
- ^ a b "Methylmalonic acidemia". MedlinePlus. National Library of Medicine. Retrieved 2024-04-30.
- ^ Villani GR, Gallo G, Scolamiero E, Salvatore F, Ruoppolo M (August 2017). ""Classical organic acidurias": diagnosis and pathogenesis". Clinical and Experimental Medicine. 17 (3): 305–323. doi:10.1007/s10238-016-0435-0. PMID 27613073.
- ^ Matsui SM, Mahoney MJ, Rosenberg LE (April 1983). "The natural history of the inherited methylmalonic acidemias". The New England Journal of Medicine. 308 (15): 857–861. doi:10.1056/NEJM198304143081501. PMID 6132336.
- ^ a b Kölker S, Garcia-Cazorla A, Valayannopoulos V, Lund AM, Burlina AB, Sykut-Cegielska J, et al. (November 2015). "The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation". Journal of Inherited Metabolic Disease. 38 (6): 1041–1057. doi:10.1007/s10545-015-9839-3. PMID 25875215.
- ^ a b "About Methylmalonic Acidemia". National Human Genome Research Institute. U.S. National Institutes of Health. Retrieved 2015-11-03.
- ^ a b c d e f g h i j k l m n o p q r s "Acidemia, Methylmalonic". NORD (National Organization for Rare Disorders). Retrieved 2015-10-29.
- ^ a b c d Alfares A, Nunez LD, Al-Thihli K, Mitchell J, Melançon S, Anastasio N, et al. (September 2011). "Combined malonic and methylmalonic aciduria: exome sequencing reveals mutations in the ACSF3 gene in patients with a non-classic phenotype". Journal of Medical Genetics. 48 (9): 602–605. doi:10.1136/jmedgenet-2011-100230. PMID 21785126.
- ^ a b c Gregg AR, Warman AW, Thorburn DR, O'Brien WE (June 1998). "Combined malonic and methylmalonic aciduria with normal malonyl-coenzyme A decarboxylase activity: a case supporting multiple aetiologies". Journal of Inherited Metabolic Disease. 21 (4): 382–390. doi:10.1023/A:1005302607897. PMID 9700595.
- ^ a b c d e f g h Sloan JL, Johnston JJ, Manoli I, Chandler RJ, Krause C, Carrillo-Carrasco N, et al. (August 2011). "Exome sequencing identifies ACSF3 as a cause of combined malonic and methylmalonic aciduria". Nature Genetics. 43 (9): 883–886. doi:10.1038/ng.908. PMC 3163731. PMID 21841779.
- ^ a b c d e f g h i j k l m n o p q "Methylmalonic acidemia". MedlinePlus Medical Encyclopedia. US National Library of Medicine. Retrieved 2024-05-01.
- ^ a b c d e f g h i j k l m n "Methylmalonic acidemia". Genetics Home Reference. US National Library of Medecine. 2015-10-26. Retrieved 2015-11-02.
- ^ a b c Saini N, Malhotra A, Chhabra S, Chhabra S (March 2015). "Methylmalonic acidemia mimicking diabetic ketoacidosis and septic shock in infants". Indian Journal of Critical Care Medicine. 19 (3): 183–185. doi:10.4103/0972-5229.152776. PMC 4366921. PMID 25810618.
- ^ a b c d e f g h i j k l Manoli I, Sloan JL, Venditti CP (2016). "Isolated Methylmalonic Acidemia". In Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). GeneReviews® [Internet]. University of Washington. PMID 20301409. NBK1231.
- ^ a b "Methylmalonic acidemia due to methylmalonyl-CoA epimerase deficiency". Orphanet. Retrieved 2024-04-27.
- ^ a b "Vitamin B12-responsive methylmalonic acidemia type cblA". Orphanet. Retrieved 2024-04-27.
- ^ "Vitamin B12-responsive methylmalonic acidemia type cblB". Orphanet. Retrieved 2024-04-27.
- ^ "Vitamin B12-unresponsive methylmalonic acidemia type mut0". Orphanet. Retrieved 2024-04-27.
- ^ "Vitamin B12-unresponsive methylmalonic acidemia type mut-". Orphanet. Retrieved 2024-04-27.
- ^ a b "Methylmalonic Aciduria Gene Panel, Varies". Mayo Clinic Laboratories. Retrieved 2024-05-26.
- ^ a b "Methylmalonic acidemia with homocystinuria, type cblJ". Orphanet. Retrieved 2024-04-27.
- ^ "Combined malonic and methylmalonic acidemia". Orphanet. Retrieved 2024-04-27.
- ^ a b "Developmental delay due to methylmalonate semialdehyde dehydrogenase deficiency". Orphanet. Retrieved 2024-04-27.
- ^ a b "Imerslund-Gräsbeck syndrome". Orphanet. Retrieved 2024-04-27.
- ^ a b "Congenital intrinsic factor deficiency". Orphanet. Retrieved 2024-05-15.
- ^ a b "Methylmalonic aciduria due to transcobalamin receptor defect". Orphanet. Retrieved 2024-04-27.
- ^ a b "Methylmalonic acidemia with homocystinuria, type cblX". Orphanet. Retrieved 2024-04-27.
- ^ Rutsch F, Gailus S, Miousse IR, Suormala T, Sagné C, Toliat MR, et al. (February 2009). "Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism". Nature Genetics. 41 (2): 234–239. doi:10.1038/ng.294. PMID 19136951. S2CID 28006539.
- ^ a b "Methylmalonic acidemia with homocystinuria type cblF". Orphanet. Retrieved 2024-04-27.
- ^ a b "Malonic aciduria". Orphanet. Retrieved 2024-04-27.
- ^ Sloan JL, Carrillo N, Adams D, Venditti CP (December 2021) [February 2008]. "Disorders of Intracellular Cobalamin Metabolism". In Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. PMID 20301503.
- ^ "Methylmalonic acidemia with homocystinuria, type cblC". Orphanet. Retrieved 2024-04-27.
- ^ Coelho D, Suormala T, Stucki M, Lerner-Ellis JP, Rosenblatt DS, Newbold RF, et al. (April 2008). "Gene identification for the cblD defect of vitamin B12 metabolism". The New England Journal of Medicine. 358 (14): 1454–1464. doi:10.1056/NEJMoa072200. PMID 18385497. S2CID 15107040.
- ^ a b "Methylmalonic acidemia with homocystinuria, type cblD". Orphanet. Retrieved 2024-04-27.
- ^ a b "Mitochondrial DNA depletion syndrome, encephalomyopathic form with methylmalonic aciduria". Orphanet. Retrieved 2024-05-11.
- ^ "Fatal infantile lactic acidosis with methylmalonic aciduria". Orphanet. Retrieved 2024-05-11.
- ^ a b "Transcobalamin deficiency". Orphanet. Retrieved 2024-04-27.
- ^ Higginbottom MC, Sweetman L, Nyhan WL (August 1978). "A syndrome of methylmalonic aciduria, homocystinuria, megaloblastic anemia and neurologic abnormalities in a vitamin B12-deficient breast-fed infant of a strict vegetarian". The New England Journal of Medicine. 299 (7): 317–323. doi:10.1056/NEJM197808172990701. PMID 683264.
- ^ "Vitamin B12 deficiency - The methylmalonic aciduria connection". The Biology Project. Department of Biochemistry and Molecular Biophysics, The University of Arizona. 31 January 2000. Archived from the original on 15 June 2018.
- ^ a b c d Baumgartner MR, Hörster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, et al. (September 2014). "Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia". Orphanet Journal of Rare Diseases. 9 (1): 130. doi:10.1186/s13023-014-0130-8. PMC 4180313. PMID 25205257.
- ^ a b c d Kumbham P, Mandava P, Zweifler RM, Kent TA, Nelson Jr SL, Gerstein BY (19 September 2022). Talavera F, Kirshner HS, Lutsep HL (eds.). "Methylmalonic Acidemia". EMedicine.
- ^ "NHGRI researchers serve up mysterious disease diagnosis - three ways". National Human Genome Research Institute. Retrieved 2024-04-28.
- ^ Gabriel MC, Rice SM, Sloan JL, Mossayebi MH, Venditti CP, Al-Kouatly HB (April 2021). "Considerations of expanded carrier screening: Lessons learned from combined malonic and methylmalonic aciduria". Molecular Genetics & Genomic Medicine. 9 (4): e1621. doi:10.1002/mgg3.1621. PMC 8123733. PMID 33625768.
- ^ a b Wehbe Z, Behringer S, Alatibi K, Watkins D, Rosenblatt D, Spiekerkoetter U, et al. (November 2019). "The emerging role of the mitochondrial fatty-acid synthase (mtFASII) in the regulation of energy metabolism". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1864 (11): 1629–1643. doi:10.1016/j.bbalip.2019.07.012. PMID 31376476.
- ^ Online Mendelian Inheritance in Man (OMIM): Methylmalonyl-CoA Epimerase Deficiency - 251120
- ^ Lee KG. "Newborn screening tests". MedlinePlus Medical Encyclopedia. Division of Neonatology, Medical University of South Carolina. Retrieved April 26, 2016.
- ^ "Newborn Screening Process". Health Resources and Services Administration (HRSA). Retrieved 2024-05-11.
- ^ Manoli I (2023-09-15). "Organic acidurias - I" (PDF). Society for Inherited Metabolic Disorders.
- ^ Held PK, Singh E, Scott Schwoerer J (February 2022). "Screening for Methylmalonic and Propionic Acidemia: Clinical Outcomes and Follow-Up Recommendations". International Journal of Neonatal Screening. 8 (1): 13. doi:10.3390/ijns8010013. PMC 8883915. PMID 35225935.
- ^ a b de Sain-van der Velden MG, van der Ham M, Jans JJ, Visser G, Prinsen HC, Verhoeven-Duif NM, et al. (2016). Morava E, Baumgartner M, Patterson M, Rahman S (eds.). "A New Approach for Fast Metabolic Diagnostics in CMAMMA". JIMD Reports. 30. Berlin, Heidelberg: Springer Berlin Heidelberg: 15–22. doi:10.1007/8904_2016_531. ISBN 978-3-662-53680-3. PMC 5110436. PMID 26915364.
- ^ de Wit MC, de Coo IF, Verbeek E, Schot R, Schoonderwoerd GC, Duran M, et al. (February 2006). "Brain abnormalities in a case of malonyl-CoA decarboxylase deficiency". Molecular Genetics and Metabolism. 87 (2): 102–106. doi:10.1016/j.ymgme.2005.09.009. PMID 16275149.
- ^ a b c Fowler B, Leonard JV, Baumgartner MR (June 2008). "Causes of and diagnostic approach to methylmalonic acidurias" (PDF). Journal of Inherited Metabolic Disease. 31 (3): 350–360. doi:10.1007/s10545-008-0839-4. PMID 18563633.
- ^ Brennerová K, Škopková M, Ostrožlíková M, Šaligová J, Staník J, Bzdúch V, et al. (December 2021). "Genetic testing is necessary for correct diagnosis and treatment in patients with isolated methylmalonic aciduria: a case report". BMC Pediatrics. 21 (1): 578. doi:10.1186/s12887-021-03067-3. PMC 8675494. PMID 34915869.
- ^ Linnell JC, Matthews DM, England JM (November 1978). "Therapeutic misuse of cyanocobalamin". Lancet. 2 (8098): 1053–1054. doi:10.1016/s0140-6736(78)92379-6. PMID 82069. S2CID 29703726.
- ^ a b c d Online Mendelian Inheritance in Man (OMIM): Methylmalonic Aciduria due to Methylmalonyl-CoA Mutase deficiency - 251000
- ^ An D, Schneller JL, Frassetto A, Liang S, Zhu X, Park JS, et al. (December 2017). "Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia". Cell Reports. 21 (12): 3548–3558. doi:10.1016/j.celrep.2017.11.081. PMC 9667413. PMID 29262333.
- ^ Martini PG, Guey LT (October 2019). "A New Era for Rare Genetic Diseases: Messenger RNA Therapy". Human Gene Therapy. 30 (10): 1180–1189. doi:10.1089/hum.2019.090. PMID 31179759.
- ^ "A Clinical Trial of a Methylmalonic Acidemia (MMA) Due to MUT Deficiency Treatment for Children and Adults". Retrieved 2024-01-04.
- ^ "Study of HST5040 in Subjects With Propionic or Methylmalonic Acidemia (HERO)". ClinicalTrials.gov. 3 January 2024. Retrieved 2024-05-24.
- ^ a b Armstrong AJ, Collado MS, Henke BR, Olson MW, Hoang SA, Hamilton CA, et al. (May 2021). "A novel small molecule approach for the treatment of propionic and methylmalonic acidemias". Molecular Genetics and Metabolism. 133 (1): 71–82. doi:10.1016/j.ymgme.2021.03.001. PMC 9109253. PMID 33741272.
- ^ "HST5040 Overview" (PDF). HemoShera. Retrieved 2024-05-26.
- ^ "A First in Human, Dose Escalation Study to Evaluate the Safety and Tolerability of BBP-671 in Healthy Volunteers and Patients With Propionic Acidemia or Methylmalonic Acidemia". ClinicalTrials.gov. 20 December 2023. Retrieved 2024-05-26.
- ^ Subramanian C, Frank MW, Sukhun R, Henry CE, Wade A, Harden ME, et al. (January 2024). "Pantothenate Kinase Activation Restores Brain Coenzyme A in a Mouse Model of Pantothenate Kinase-Associated Neurodegeneration". The Journal of Pharmacology and Experimental Therapeutics. 388 (1): 171–180. doi:10.1124/jpet.123.001919. PMID 37875310.
- ^ Oberholzer VG, Levin B, Burgess EA, Young WF (October 1967). "Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis". Archives of Disease in Childhood. 42 (225): 492–504. doi:10.1136/adc.42.225.492. PMC 2019805. PMID 6061291.
- ^ a b c Han L, Wu S, Han F, Gu X (Jun 15, 2015). "Insights into the molecular mechanisms of methylmalonic acidemia using microarray technology". International Journal of Clinical and Experimental Medicine. 8 (6): 8866–8879. PMC 4538064. PMID 26309541.
- ^ Chandler RJ, Zerfas PM, Shanske S, Sloan J, Hoffmann V, DiMauro S, et al. (April 2009). "Mitochondrial dysfunction in mut methylmalonic acidemia". FASEB Journal. 23 (4): 1252–1261. doi:10.1096/fj.08-121848. PMC 2660647. PMID 19088183.
- ^ a b c Underhill HR, Hahn SH, Hale SL, Merritt JL (December 2013). "Asymptomatic methylmalonic acidemia in a homozygous MUT mutation (p.P86L)". Pediatrics International. 55 (6): e156–e158. doi:10.1111/ped.12195. PMID 24330302. S2CID 27495325.
