ORF9b
| Betacoronavirus lipid binding protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
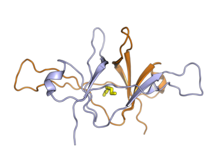 The X-ray crystallography structure of the SARS-CoV ORF9b protein dimer, showing the lipid molecule in the central cavity (yellow). From PDB: 2CME.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | bCoV_lipid_BD | ||||||||
| Pfam | PF09399 | ||||||||
| InterPro | IPR018542 | ||||||||
| |||||||||
ORF9b (formerly sometimes called ORF13) is a gene that encodes a viral accessory protein in coronaviruses of the subgenus Sarbecovirus, including SARS-CoV and SARS-CoV-2. It is an overlapping gene whose open reading frame is entirely contained within the N gene, which encodes coronavirus nucleocapsid protein.[2][3][4] The encoded protein is 97 amino acid residues long in SARS-CoV[2][3] and 98 in SARS-CoV-2,[4] in both cases forming a protein dimer.
Nomenclature
There has been inconsistency in the nomenclature used for this gene in the scientific literature. In some work on SARS-CoV, it has been referred to as ORF13. It has also sometimes been referred to as ORF9a, resulting in a downstream ORF of 76 codons in SARS-CoV, also overlapping with the N gene, being designated ORF9b. The recommended nomenclature refers to the longer ORF as 9b and the downstream, shorter ORF as ORF9c.[5]
Structure
The ORF9b protein is 97 amino acid residues long in SARS-CoV[2][3] and 98 in SARS-CoV-2.[4] It forms a beta sheet-rich homodimer with a hydrophobic cavity in the center that binds lipids.[2][3][4] The lipid-binding cavity may serve as an unusual mechanism for anchoring the protein to membranes.[1]
A fragment of the SARS-CoV-2 ORF9b protein has been structurally characterized in a protein complex with Tom70 in which ORF9b forms an alpha helix rather than the beta-sheet structure observed in isolation.[6] This fold switching behavior is also consistent with bioinformatics predictions and may also occur for the SARS-CoV homolog.[7]
Expression and localization
ORF9b is one of two overlapping genes fully contained within the open reading frame of the N gene encoding coronavirus nucleocapsid protein, the other being ORF9c. ORF9b is expressed by ribosome leaky scanning from its bicistronic subgenomic RNA.[2][3][8] Unlike its neighbor ORF9c, its length is well conserved in sarbecoviruses and there is strong evidence it is a functional protein-coding gene.[9]
In SARS-CoV, the protein is localized to the endoplasmic reticulum (ER)[3] and to intracellular vesicles.[2][1] It does not have a nuclear localization sequence but can enter the cell nucleus by passive diffusion; it does however have a nuclear export sequence for exit from the nucleus.[2][3] In SARS-CoV-2, it is reportedly associated with the mitochondrial membrane.[4]
Function
The function of the ORF9b protein is not well characterized. It is not essential for viral replication.[2]
Viral protein interactions
The ORF9b protein has been reported to interact with a number of other viral proteins, including ORF6, non-structural protein 5, non-structural protein 14, and coronavirus envelope protein.[2] It has been detected in mature SARS-CoV virions and thus may be a minor viral structural protein.[2][3][8]
Host cell effects
The ORF9b protein may be involved in modulating the host's immune system response. The SARS-CoV-2 protein has been reported to suppress interferon response via its interactions with Tom70, a component of the mitochondrial translocase of the outer membrane (TOM) complex.[6][10]
References
- ^ a b c Meier C, Aricescu AR, Assenberg R, Aplin RT, Gilbert RJ, Grimes JM, et al. (July 2006). "The crystal structure of ORF-9b, a lipid binding protein from the SARS coronavirus". Structure. 14 (7): 1157–1165. doi:10.1016/j.str.2006.05.012. PMC 7126280. PMID 16843897.
- ^ a b c d e f g h i j Liu DX, Fung TS, Chong KK, Shukla A, Hilgenfeld R (September 2014). "Accessory proteins of SARS-CoV and other coronaviruses". Antiviral Research. 109: 97–109. doi:10.1016/j.antiviral.2014.06.013. PMC 7113789. PMID 24995382.
- ^ a b c d e f g h McBride R, Fielding BC (November 2012). "The role of severe acute respiratory syndrome (SARS)-coronavirus accessory proteins in virus pathogenesis". Viruses. 4 (11): 2902–2923. doi:10.3390/v4112902. PMC 3509677. PMID 23202509.
- ^ a b c d e Redondo N, Zaldívar-López S, Garrido JJ, Montoya M (7 July 2021). "SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns". Frontiers in Immunology. 12: 708264. doi:10.3389/fimmu.2021.708264. PMC 8293742. PMID 34305949.
- ^ Jungreis I, Nelson CW, Ardern Z, Finkel Y, Krogan NJ, Sato K, et al. (June 2021). "Conflicting and ambiguous names of overlapping ORFs in the SARS-CoV-2 genome: A homology-based resolution". Virology. 558: 145–151. doi:10.1016/j.virol.2021.02.013. PMC 7967279. PMID 33774510.
- ^ a b Gao X, Zhu K, Qin B, Olieric V, Wang M, Cui S (May 2021). "Crystal structure of SARS-CoV-2 Orf9b in complex with human TOM70 suggests unusual virus-host interactions". Nature Communications. 12 (1): 2843. Bibcode:2021NatCo..12.2843G. doi:10.1038/s41467-021-23118-8. PMC 8121815. PMID 33990585.
- ^ Porter LL (August 2021). "Predictable fold switching by the SARS-CoV-2 protein ORF9b". Protein Science. 30 (8): 1723–1729. doi:10.1002/pro.4097. PMC 8242659. PMID 33934422.
- ^ a b Xu K, Zheng BJ, Zeng R, Lu W, Lin YP, Xue L, et al. (June 2009). "Severe acute respiratory syndrome coronavirus accessory protein 9b is a virion-associated protein". Virology. 388 (2): 279–285. doi:10.1016/j.virol.2009.03.032. PMC 7103405. PMID 19394665.
- ^ Jungreis I, Sealfon R, Kellis M (May 2021). "SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes". Nature Communications. 12 (1): 2642. Bibcode:2021NatCo..12.2642J. doi:10.1038/s41467-021-22905-7. PMC 8113528. PMID 33976134.
- ^ Jiang HW, Zhang HN, Meng QF, Xie J, Li Y, Chen H, et al. (September 2020). "SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70". Cellular & Molecular Immunology. 17 (9): 998–1000. doi:10.1038/s41423-020-0514-8. PMC 7387808. PMID 32728199.

