para-Bromoamphetamine
Appearance
This article needs additional citations for verification. (November 2009) |
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
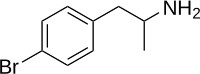
| Formula | C9H12BrN |
| Molar mass | 214.106 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
para-Bromoamphetamine (PBA), also known as 4-bromoamphetamine (4-BA), is an amphetamine derivative which acts as a serotonin-norepinephrine-dopamine releasing agent (SNDRA) and produces stimulant effects.
Another related compound is para-bromomethamphetamine (PBMA) known by the codename V-111.[1]
Pharmacology
PBA has been found to be a monoamine oxidase A (MAO-A) inhibitor, with an IC50 of 1,500 nM.[2]
Neurotoxicity
Like most other para-substituted amphetamines, PBA can be neurotoxic and may deplete the brain of 5-hydroxyindoles for at least a week.[3]
See also
- Substituted amphetamines
- 4-Bromomethcathinone (4-BMC)
- 4-Fluoroamphetamine (4-FA)
- para-Chloroamphetamine (PCA)
- para-Iodoamphetamine (PIA)
References
- ^ Magyar K, Tekes K, Zólyomi G, Szüts T, Knoll J (1981). "The fate of p-bromo-methylamphetamine (V-111) in the body". Acta Physiologica Academiae Scientiarum Hungaricae. 57 (3): 285–307. PMID 7304194.
- ^ Reyes-Parada M, Iturriaga-Vasquez P, Cassels BK (2019). "Amphetamine Derivatives as Monoamine Oxidase Inhibitors". Front Pharmacol. 10: 1590. doi:10.3389/fphar.2019.01590. PMC 6989591. PMID 32038257.
- ^ Fuller RW, Baker JC, Perry KW, Molloy BB (October 1975). "Comparison of 4-chloro-, 4-bromo- and 4-fluoroamphetamine in rats: drug levels in brain and effects on brain serotonin metabolism". Neuropharmacology. 14 (10): 739–46. doi:10.1016/0028-3908(75)90099-4. PMID 1196472. S2CID 9620299.
