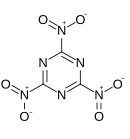
Trinitrotriazine
Appearance
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,4,6-Trinitro-1,3,5-triazine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3N6O6 | |||
| Molar mass | 216.069 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trinitrotriazine, or 2,4,6-trinitro-1,3,5-triazine, is a theoretical explosive compound. Synthesis of this compound has been elusive despite its simple structure,[1][2] as conventional nitration of triazine becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by trimerisation of nitryl cyanide.[3] The precursor nitryl cyanide was first synthesized by Rahm et al. in 2014.[4]
Trinitrotriazine has a neutral oxygen balance, potentially making it a very powerful explosive, though calculations predict it would be fairly unstable and inferior to the related compound 3,6-dinitro-1,2,4,5-tetrazine.[5]
See also
- RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine)
References
- ^ G. D. Hartman, R. D. Hartman and J. E. Schwering. Tetrahedron Letters. 1983; 24: 1011.
- ^ M. D. Coburn, C. L. Coon, H. H. Hayden and A. R. Mitchell. Synthesis. 1986: 490.
- ^ Korkin AA. Bartlett RJ. Theoretical Prediction of 2,4,6-Trinitro-1,3,5-triazine (TNTA). A New, Powerful, High-Energy Density Material? Journal of the American Chemical Society. 1996; 118: 12244-12245.
- ^ Rahm, M., Bélanger-Chabot, G., Haiges, R. and Christe, K. O. (2014), Nitryl Cyanide, NCNO2. Angew. Chem. Int. Ed., 53: 6893–6897
- ^ Jinshan Li. An Ab Initio Theoretical Study of 2,4,6-Trinitro-1,3,5-Triazine, 3,6-Dinitro-1,2,4,5-Tetrazine, and 2,5,8-Trinitro-Tri-s-Triazine, more commonly known as RDX. Propellants, Explosives, Pyrotechnics. December 2008; 33(6):443-447.