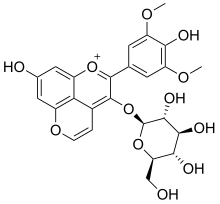
Vitisin B (pyranoanthocyanin)
Appearance
| |
| Names | |
|---|---|
| IUPAC name
3-(β-D-Glucopyranosyloxy)-4′,7-dihydroxy-3′,5′-dimethoxypyrano[4′′,3′′,2′′:4,5]flavylium
| |
| Systematic IUPAC name
8-Hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-pyrano[4,3,2-de][1]benzopyran-1-ylium | |
| Other names
Pyranomalvidin-3-glucoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H25O12+ | |
| Molar mass | 517.45 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vitisin B is a natural phenol found in red wines.[1][2] It is a pyranoanthocyanin.[3][4][5]
See also
References
- ^ Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Suzana Lucy Nixdorf and Isidro Hermosín-Gutiérrez, Analytica Chimica Acta, Volume 659, Issues 1-2, 5 February 2010, Pages 208-215, doi:10.1016/j.aca.2009.11.058
- ^ Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. A. Morata, F. Calderón, M.C. González, M.C. Gómez-Cordovés and J.A. Suárez, Food Chemistry, Volume 100, Issue 3, 2007, Pages 1144-1152, doi:10.1016/j.foodchem.2005.11.024
- ^ A novel synthetic pathway to vitisin B compounds. Joana Oliveira, Victor de Freitas and Nuno Mateus, Tetrahedron Letters, Volume 50, Issue 27, 8 July 2009, Pages 3933-3935, doi:10.1016/j.tetlet.2009.04.072
- ^ Charge equilibria and pK values of 5-carboxypyranomalvidin-3-glucoside (vitisin A) by electrophoresis and absorption spectroscopy. Robert E. Asenstorfer and Graham P. Jones, Tetrahedron, Volume 63, Issue 22, 28 May 2007, Pages 4788-4792, doi:10.1016/j.tet.2007.03.052
- ^ Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. Romero C. and Bakker J., International journal of food science & technology, 2000, vol. 35, no. 1, pp. 129-140, INIST 1283952
