Wikipedia:Reference desk/Archives/Science/2011 July 25
| Science desk | ||
|---|---|---|
| < July 24 | << Jun | July | Aug >> | July 26 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 25
Line of cooling, line of heating
Is there any map of the USA which shows a line to the north of which you do not require air conditioning in the summer, and to the south you do? And similarly is there a map with a line to the south of which you do not require winter heating? 2.101.4.222 (talk) 09:05, 25 July 2011 (UTC)
- What temperatures would "require" air conditioning or heating? I have no air conditioning while the guy I work with has it. If I had to guess, I'd say more people around me don't have air conditioning than do. So, would this requirement line be north of me or south? I would suspect that if you were to draw a vertical line on a map and then poll the households along that line, there would be a gradient for both heating and cooling that is more or less a smooth decline or accumulation depending on which way you go up or down that line. You will at some point reach 0% of homes that has either heating or cooling. So would this be your line of requirement? 0%? Dismas|(talk) 09:31, 25 July 2011 (UTC)
- 50% of households would be an appropriate line. 2.101.4.222 (talk) 09:44, 25 July 2011 (UTC)
- That's an iffy way to try to collect that data. While air conditioning is very pleasant to have in warm climates, it's not essential for most individuals the way that home heating is. (In the sense of 'your water pipes will freeze solid, then you will die of hypothermia'.) The "50% air conditioned" line will be skewed very heavily by demographics—regions with lower household incomes will be less air-conditioned than higher-income regions. You'll probably also see significant rural-suburban-urban splits. (Dismas' suggestion to look for a 0% air-conditioner penetrance is interesting, but would probably rule out the entire United States. As of 2002, something like 30% of households in Canada had an air conditioner, and that number was steadily trending upwards.)
- What you're probably looking for is something like a map of heating degree days (and cooling degree days). That article has both maps for the United States, and a pretty good explanation of what the numbers represent. TenOfAllTrades(talk) 13:50, 25 July 2011 (UTC)
- Many homes in the Southeastern United States do not have central heating, even though temperatures occasionally fall below freezing. You could even say the line is variable between different years. ~AH1 (discuss!) 15:51, 25 July 2011 (UTC)
- Climate on a large continent is not that simple. For example, I live on a state that borders Canada, and it was 102 degrees Fahrenheit last week. I certainly wouldn't want to live here without air conditioning. Washington state, at the same latitude, rarely gets that hot on the coasts, but does inland. So you wouldn't end up with a straight line. thx1138 (talk) 16:32, 25 July 2011 (UTC)
- I wasnt suggesting that the line would be straight. 92.29.124.70 (talk) 13:17, 30 July 2011 (UTC)
- engineers don't really calculate this; it tends to be trial and error. the calculation of thermal comfort is ASHRAE 55; see LEED discussion: [1]. you could combine the weather data and thermal comfort to get a map, but there would be lines of percentage dis-comfort. (or you could use the plant zone map). 98.163.75.189 (talk) 16:10, 30 July 2011 (UTC)
- I wasnt suggesting that the line would be straight. 92.29.124.70 (talk) 13:17, 30 July 2011 (UTC)
Better US climate than London
Most of the US, compared to Britain, gets extremely hot in the summer, and extremely cold in the winter. By comparison the average winter temperature in London is above freezing with hardly any snow, and only during infrequent summer heat waves would you appreciate air-conditioning in the home. Nor does it rain much (contrary to US stereotypes) with around 20 inches of precipitation a year.
Which places in the US have a better climate than London? 2.101.4.222 (talk) 09:13, 25 July 2011 (UTC)
- Could you better define "better"? I quite like where I live and we regularly get a few feet of snow each winter. To me, that's better than London. But it seems as though you're looking for a place where the temperature range isn't very wide and where there is very little precipitation. Am I right? Dismas|(talk) 09:23, 25 July 2011 (UTC)
- Principally where it is not extremely hot in summer or extremely cold in winter, and preferably with around 20 inches or less of precipitation. 2.101.4.222 (talk) 09:43, 25 July 2011 (UTC)
- Again, you'll have to better explain what you mean by "extremely". My niece in Georgia would likely tell me that anything below 20F is extremely cold while I don't mind these temperatures. Moving along though... Well, I don't have a map with a pin in it for you but can provide some links to maps so that you can figure it out on your own... This pdf shows the average rainfall in 2001 as well as average temperature but unfortunately not the range. NOAA has a collection of maps that are relevant. As well as How Stuff Works. This site seems very handy as well. Dismas|(talk) 09:54, 25 July 2011 (UTC)
- Principally where it is not extremely hot in summer or extremely cold in winter, and preferably with around 20 inches or less of precipitation. 2.101.4.222 (talk) 09:43, 25 July 2011 (UTC)
- Honolulu may be a bit too warm for you, but it never gets unbearably hot. And you can fine-tune average rain fall to your liking by picking the right place between the mountains and the sea. --Wrongfilter (talk) 10:35, 25 July 2011 (UTC)
- San Diego has a reputation for an extraordinarily equable climate. See the article Climate of San Diego. Deor (talk) 12:08, 25 July 2011 (UTC)
- You might also try Seattle, which does not frequently get much snow compared to higher elevations, though there is an inherent earthquake risk. ~AH1 (discuss!) 15:48, 25 July 2011 (UTC)
- Drawback to Seattle is it gets around 40 inches of rain a year compared to 20-25 for London, but they do appear broadly similar. Googlemeister (talk) 15:56, 25 July 2011 (UTC)
- You might also try Seattle, which does not frequently get much snow compared to higher elevations, though there is an inherent earthquake risk. ~AH1 (discuss!) 15:48, 25 July 2011 (UTC)
- San Francisco? --Jayron32 16:04, 25 July 2011 (UTC)
- Yes, and neighboring parts of California. Los Angeles and San Diego also have nice parts, but if you get more than a mile or so from the beach they are pretty hot. Looie496 (talk) 21:20, 25 July 2011 (UTC)
- The inquirer apparently means a mild climate, which means on the coast. NYC, Long Island and Cape Cod are not bad. I personally prefer a winter where it is cold enough to ice skate and a summer warm enough to swim in the ocean without shivering. Most articles on cities give their mean monthly temperatures. μηδείς (talk) 01:11, 26 July 2011 (UTC)
- If NYC means New York City, then I thought that it was unbearably hot and humid in summer and below freezing in winter? 92.28.250.101 (talk) 10:40, 28 July 2011 (UTC)
- The inquirer apparently means a mild climate, which means on the coast. NYC, Long Island and Cape Cod are not bad. I personally prefer a winter where it is cold enough to ice skate and a summer warm enough to swim in the ocean without shivering. Most articles on cities give their mean monthly temperatures. μηδείς (talk) 01:11, 26 July 2011 (UTC)
I'm getting the impression that apart from San Francisco and San Diego, everywhere in the US is either unbearably hot in summer or unbearably cold in winter, and in many places both, but never neither. 92.29.113.104 (talk) 22:56, 29 July 2011 (UTC)
Phase diagrams
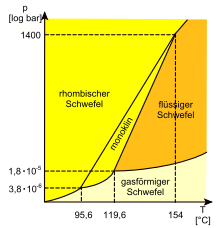
Does anyone know where to find phase diagrams for phosphorus and sulfur, including the allotropes and molecular species present? At what temperature does octathiocane autoignite, does it burst into flame before it melts? Plasmic Physics (talk) 09:58, 25 July 2011 (UTC)
- I have the phase map for sulfur it could be found here just before "4 phases at the extremes"--Irrational number (talk) 14:38, 25 July 2011 (UTC)
- I typically do an image search on "critical point" and the name of the substance for phase diagrams. Octathiocane says it decomposes at 115 Celsius. 99.2.148.119 (talk) 19:13, 25 July 2011 (UTC)

- For sulfur there is a German phase diagram available on commons. I was converting it to .svg form, but the quality was so low that I abandoned the attempt. It is actually much more complex as at higher temperatures there are mixtures of molecules, including trisulfur and other allotropes of sulfur such as S6 and S2 in the gas. What is there depends on the thermal history of the mix. There is a chart for Phosphorus but not a phase diagram here. Graeme Bartlett (talk) 10:22, 26 July 2011 (UTC)
- Thanks, good diagram for sulfur, it's a good thing I read german. So, you're saying that the exact composition of sulfur at equilibrium may vary depending on the thermodynamic pathway? Even if several molecular species' thermodynamic domains overlap, there should be boundaries where they do not exist under equilibrium? For instance, disulfur what are it's absolute constraints? Plasmic Physics (talk) 15:28, 26 July 2011 (UTC)
- I put up an English version at File:Sulfur phase diagram.svg. (The subscript "8" is still off... it seems like you never know what the svg files will look like after upload, and I had to resort to a Greek en-spacing character to get this much) Wnt (talk) 18:24, 26 July 2011 (UTC)
- Where does gamma-sulfur fit in this diagram? It is also monoclinic like beta-sulfur. Plasmic Physics (talk) 00:18, 28 July 2011 (UTC)
- Wow. No idea. [2] calls it "notoriously unstable and erratic". Yet it is also the mineral rosickyite, according to the allotropes of sulfur article! (no, alas, that mineral link just goes back to sulfur :( ) We may need a real chemist (or geologist?) to answer this one. Wnt (talk) 18:37, 29 July 2011 (UTC)
Hi all!
IPNI says he's not a taxon authority. A WP:PROD is on foot, and I can't find anything to contest this with, so over to you, the experts.
As always, thanks for all your help and guidance.--Shirt58 (talk) 12:23, 25 July 2011 (UTC)
- Whether he is notable or not, his article appears to contain no assertion of notability, which is necessary. You may want WP:N/N instead of the reference desk. 99.2.148.119 (talk) 21:04, 25 July 2011 (UTC)
question about origin of life
I often hear that people and even some scientific programs say that all life on earth is evolve from a single living cell but it doesn't make sense to me because:
a.there is a very small chance of survival for that single cell.
b.the chemical process that led to the formation of life couldn't have happened only one time and produced one cell only because in nature chemical reactions happen in great numbers.
is it a misconception? was it one cell or one "kind" of cell( or even more)?did it only happen in one place or it happened in many places worldwide?thanks in advance.--Irrational number (talk) 14:26, 25 July 2011 (UTC)
- Life evolved from single-celled organisms. Not one specific single cell. Zzubnik (talk) 14:36, 25 July 2011 (UTC)
- (ec) Your questions are probably answered in this section, current models of abiogenesis. We don't have great certainty, but it is speculated that abiogenesis may have occurred more than once, "merging" independently into the earliest archaeobacteria. Nimur (talk) 14:40, 25 July 2011 (UTC)
- Much as the mitochondria in each of our cells seems to have started out as a separate organism. See Mitochondria#Origin. StuRat (talk) 15:30, 25 July 2011 (UTC)
- Also, the molecules essential for life evolved from basic molecules long before single cell organisms arose. In a NGC documentary, it was mentioned that some scientists are looking into the possibility that complex molecules evolved from simple molecules inside comets. The idea is that to get complex molecules from simple molecules before you have the protective cell environment that contains all the other enzymes that keeps everything stable, requires low temperatures that prevents thermal equilibrium from being reached.
- There are then chemical reactions between molecules that happen to be very close to each other, driven by exposure to cosmic rays and X rays, but the molecules cannot move around to react with other molecules that are further away, and that allows the creation of very unstable molecules, some of which may happen to be a part of some molecule essential for life.
- When the comet is kicked out of its orbit in the Oort cloud, it periodically comes closer to the Sun. When part of the comet melts, some of the unstable molecules are able to move around, most will react to form simple compounds like methane and H2O, or simply disintegrate, but by accident some very unstable molecules will combine to form more stable larger molecules. When the comet moves away from the Sun, that will then limit the reactions to between neighbors only, but now these also involve the larger molecules that are the most stable combination of two or more molecules that were cooked up the previous time.
- After millions of years, the very complex molecules essential for life can be build up step by step this way. Count Iblis (talk) 15:10, 25 July 2011 (UTC)
- You may also be interested in epigenetics and viral fossil. ~AH1 (discuss!) 15:45, 25 July 2011 (UTC)
- The idea that all current life descends from a single cell does not imply that that cell was the only organism alive at the time or that abiogenesis happened only once. It only means that all other past life forms from different lineages possible different abiogenesis events are losers and have become extinct, unable to compete with the single remaining life lineage. Dauto (talk) 16:20, 25 July 2011 (UTC)
- To put it another way - even if all life on earth is descended from one single-celled organism, it doesn't mean that was the only organism that was alive at the time. thx1138 (talk) 16:34, 25 July 2011 (UTC)
In the above discussion at present, "cell" could be replaced in many instances by "molecule" and it would be just as valid. The fact that modern cells are symbiotic with the mitochondria they contain suggests that life did not descend from a single cell, and by analogy I think it much more likely that it didn't descend from a single molecule either. Complex organic molecules, some with the properties necessary for self-replication, including long polymers, many of the nucleotides and amino acids, occur naturally when the atmosphere of the pre-biotic Earth from the fossil record is exposed to lightning. Please see Miller–Urey experiment. 99.2.148.119 (talk) 17:55, 25 July 2011 (UTC)
- The OP is correct that the chance of survival of the earliest life form is very small. We can only observe that the Universe is VERY BIG, maybe infinitely so, so that somewhere sometime even the most unlikely circumstances will arise. We don't know in how many places at how many times life has arisen, only that it arose here on Earth. Our conclusions must be that 1) we need to take care of the full diversity of living things on Earth because they are unique and irreplaceable, and 2) looking for signs of extraterrestrial life is not a hopeless quest. Cuddlyable3 (talk) 18:28, 25 July 2011 (UTC)
- Similarly, almost all humans are the result of millions of possible sperm and thousands of possible eggs, so the likelihood that their particular genome would have resulted in a person was very small before it actually happened. 99.2.148.119 (talk) 19:17, 25 July 2011 (UTC)
- The OP is correct that the chance of survival of the earliest life form is very small. We can only observe that the Universe is VERY BIG, maybe infinitely so, so that somewhere sometime even the most unlikely circumstances will arise. We don't know in how many places at how many times life has arisen, only that it arose here on Earth. Our conclusions must be that 1) we need to take care of the full diversity of living things on Earth because they are unique and irreplaceable, and 2) looking for signs of extraterrestrial life is not a hopeless quest. Cuddlyable3 (talk) 18:28, 25 July 2011 (UTC)
The replicating-molecule first theory is deeply flawed. Lipids mixed with water spontaneously form miscelles (think bubble) which spontaneously split when they have grown large enough. For the cellular metabolism first theory see Stuart Kauffman. μηδείς (talk) 19:22, 25 July 2011 (UTC)
- I agree lipids and many other kinds of molecules must have been involved in the first cell. This is an excellent illustrated explanation. We may never know if there was only one first cell by any particular definition, but there were certainly an abundance of protocells. 99.2.148.119 (talk) 21:00, 25 July 2011 (UTC)
Kaufmann's point is that the cell membrane preceded the existence of DNA or RNA, that boundedness precedes replication. I would oversimplify the membrane-first theory as saying that soap bubbles, oil blebs, or sea foam stirred by waves grow and divide spontaneously. The ancient oceans were full enough of complex molecules that once some of these bubbles had a set of molecules (Again, see Stuart Kauffman's Origins of Order) which gave them an advantage at replicating and absorbing more such molecules across their membranes. Kauffman speaks of the primacy of the metabolistic systems. Basically, if the question interests you and you want to say anything on the subject of the origin of life that is not totally naive you have to read him. μηδείς (talk) 21:27, 25 July 2011 (UTC)
- Personally I am fond of the idea that life could have existed as an open meshwork of RNA strands carrying all of the constituents of metabolism as covalently bound functional groups; while the soap bubble idea seems facile I just am too skeptical that RNA could effectively regulate an intact membrane before peptides (NRPS) were invented. I prefer the idea that proteins could have evolved as directly processed RNA strands - the biosynthesis of histidine from PRPP (not in Wikipedia as of yet) provides a model. So I like to think of cells as the massively evolved products that result long after biofilms of RNA have surrounded themselves with proteins and crude "cuticles" of phospholipids protecting the surfaces on which they lie. But this isn't an established model, and I don't know if we can ever know what happened really. Wnt (talk) 18:31, 26 July 2011 (UTC)
Aquarium filters
how does filters in fish aquariums work how does they provide oxygen in the tank ..i want a scientific answer i mean all the processes that takes place in it (osmosis, etc) — Preceding unsigned comment added by 175.110.58.208 (talk) 16:44, 25 July 2011 (UTC)
- I don't think filters do provide oxygen. That's done by bubbling air through the water. The filter removes things from the water, it doesn't add things. StuRat (talk) 16:50, 25 July 2011 (UTC)
- For the filters, see activated carbon and biological filter. ~AH1 (discuss!) 16:54, 25 July 2011 (UTC)
- Also note that for a small enough aquarium, say a goldfish bowl, enough oxygen is absorbed from the air at the surface (goldfish are also more tolerant of low oxygen levels). For larger aquariums, bubbling air through the water increases the surface area of the contact, and this allows more oxygen to be absorbed. StuRat (talk) 17:09, 25 July 2011 (UTC)
- The goldfish has a special trick to survice in bowls. It's called the Labyrinth organ, which allow the goldfish to get oxygen by gulping air. The oxygen in the bowl itself may not be sufficient. EverGreg (talk) 22:46, 25 July 2011 (UTC)
- There are different types of filters most use activated carbon or artificial fibers or some combination. These are usually driven by an air pump which has the side effect of releasing bubbles in the water or a water pump which aids in mixing enough to aerate the water. There is no direct connection between the filtering action of the fibers or carbon and the aeration itself. μηδείς (talk) 19:31, 25 July 2011 (UTC)
- The main function of the aquarium filter is not to provide oxygen. The sponge-like filters provide a surface for beneficial bacteria that break down waste products (fish poo) in the aquarium water. The pump is there to help oxygen-rich water and waste circulate through the filters, but by creating movement in the water, the pump also help oxygen being exchanged with the air above the water surface. Depending on pH, the waste result in Ammonia (NH3) in the water, which bacteria will break down into poisonous nitrite. The filter bacteria neutralize the nitrite by furter transforming it to nitrate, which is less poisonous. The nitrate may subsequently become nutrition for plants and algae in the aquarium. (And some fish eat algae, setting up a simple but fascinating eco-system) Without a filter, fish in a typical aquarium will suffer poisoning after a couple of weeks. EverGreg (talk) 22:40, 25 July 2011 (UTC)
Lead screws
Lead screws for machines are normally made by lathe. But how was the lead screw for the first lathe made?--78.148.137.217 (talk) 18:29, 25 July 2011 (UTC)
- Various methods, including manual methods, for making screw threads are discussed at the threading (manufacturing) article. -- Finlay McWalter ☻ Talk 18:35, 25 July 2011 (UTC)
- One of the best links for answering your question is Screw-cutting lathe > History. It all started out with hand-whittled and hand-filed shapes, and progressed from there, gradually increasing accuracy and precision, in bootstrapping fashion (using one iteration to make future iterations, and finding clever ways to detect and reduce the error along the way). After reading that link, you may wonder how it is that they moved from fairly accurate to extremely accurate screw leads. I don't think Wikipedia has good coverage yet of the answers to that, but the answers involve things like using dividing engines to make high-accuracy gears, which were then arranged into gear trains, yielding precise gear ratios. These, combined with rack and pinion setups and slide rests, allowed them to cut ever-more-accurate leadscrews and build ever-more-accurate lathes (bootstrapping upward). The pinion gear pitch could be accurately controlled with linear measurements and careful filing with magnifying glasses, etc; and the flatness of the slide rests could be controlled with the three-plate method of generating flat surfaces [see Joseph Whitworth]). All of this evolved into metrology, which later also incorporated optics, electronics, and other technologies. — ¾-10 22:59, 25 July 2011 (UTC)
- Interesting answer, but I dont see how accuracy can be 'bootstrapped' upward: how does it work? What I can believe is that faily accurate lead screws could have been made using an accurate rack and pinion system which could be made by hand. Thats my opinion anyway.
--GearCutter (talk) 12:02, 26 July 2011 (UTC)
virtual particles.
In simple terms do virtual particles pop into existence in a vacuum because of an energy field?
If there was a vacuum and no field would there be no particles? (or is it not possible to have a vacuum with no energy field?) — Preceding unsigned comment added by 92.23.138.8 (talk) 19:47, 25 July 2011 (UTC)
- The existance of anything at all creates a field. Imagine if, in the entire universe, there existed a single electron. That electron has now created an electric field which extends to infinity in all dimensions of spacetime. It would be impossible to suppose a universe in which anything at all existed, but which did not have any fields. --Jayron32 19:58, 25 July 2011 (UTC)
- Yes. However in quantum field theory, even the minimum-energy vacuum state gives rise to virtual particles. Looie496 (talk) 21:13, 25 July 2011 (UTC)
- In simple terms, the answer is no, there is no such a thing as an energy field. Dauto (talk) 21:31, 25 July 2011 (UTC)
So Gravitational fields , electric/magnetic fields are not energy fields? Are they not fields that store energy? — Preceding unsigned comment added by 92.23.138.8 (talk) 21:49, 25 July 2011 (UTC)
- Technically they are force fields, but they don't store force, they transmit it. Sadly "force field" has been so overused in science fiction that I doubt anyone can think of that phrase without imagining an invisible barrier that lights up when things collide with it. 99.2.148.119 (talk) 22:38, 25 July 2011 (UTC)
- No physicist calls them energy fields. They are simply called fields. Those "force" fields will have virtual particles but so will the "matter" fields such as the electron field for instance. Dauto (talk) 01:28, 26 July 2011 (UTC)
Sleep of the ancients (and the not so ancient)
While having a discussion about family history, my father noted that my grandparents would not sleep through the entire night. They would go to bed a few hours after sundown, and they would always wake up at around 1 or 2-ish in the morning for a short "break from sleep" (as it where) to have a chat, do some busywork, etc , then go back to sleep until sunrise. My father guessed that it was because my grandparents lived in a very rural part of China (i.e. there is no electricity or modern lighting for miles). Is this sleep pattern widespread? and why don't we do it anymore? — Preceding unsigned comment added by 99.254.130.157 (talk) 21:41, 25 July 2011 (UTC)
- If I had to guess, I would say that interrupted sleep in two 3-4 hour naps per day is about as common as shift work. It is all what you are used to, and with modern air conditioning, it's often just as comfortable to stay up late. 99.2.148.119 (talk) 22:42, 25 July 2011 (UTC)
- Here is an NY Times article on sleep where they discuss the two sleep pattern. - Akamad (talk) 01:29, 26 July 2011 (UTC)
This is covered in segmented sleep. Personally I find this interesting, because even though I usually don't take a break from sleep, there are differences between the first few hours and the rest - for example, the first part seems deeper, dreamless, less voluntary, more affected by diet, and can proceed despite noise (such as a television); when awakened it feels disorienting, as if my "center of consciousness" has descended. Dreams seem reserved for the light, semi-waking second part. It makes me wonder if the two periods of sleep are really fundamentally different, with different purposes. The article Sleep shows something somewhat akin to this, with more N3 and N4 sleep early on, but minus such extravagant claims. Wnt (talk) 04:27, 26 July 2011 (UTC)
- A quick scan of the literature on Geriatric sleep patterns confirms what I thought I'd heard a few years ago: we don't need as much sleep as we get older as we did when we were younger. As an aside, my own grandparents (both have been dead now for over 30 years and were in their late 80s when they died) never slept well at night: I put that down to the fact that my grandmother was a florist and had to be at the market at 5 am to choose the day's flowers, and my grandfather worked in demolition and had early starts there. That's what made me curious as to whether it was just them, or a wider spread phenomenon. It seems to be the latter. --TammyMoet (talk) 09:09, 26 July 2011 (UTC)
- Polyphasic sleep also discusses the "ancestral sleep state". ~AH1 (discuss!) 00:41, 29 July 2011 (UTC)
- I have the privelidge of being able to get up or go to bed whenever I want, and I've also noticed my sleep often falling into two parts with waking in the middle. Perhaps this is what humans evolved to do naturally, or perhaps it is over-sleeping. 92.29.124.70 (talk) 13:21, 30 July 2011 (UTC)
O42-
If the O2- ion is called the oxide ion, the O22- ion is called the peroxide ion, and the O32- ion is called the ozonide ion, what is the O42- ion called? And what about the O52- and O62- ions? Whoop whoop pull up Bitching Betty | Averted crashes 22:22, 25 July 2011 (UTC)
- I am not sure such species exist. How would O62- be bound? 99.2.148.119 (talk) 22:58, 25 July 2011 (UTC)
- It would be a chain of six oxygen ions connected by single bonds, with those at the ends carrying one negative charge each (-O-O-O-O-O-O-). Whoop whoop pull up Bitching Betty | Averted crashes 23:11, 25 July 2011 (UTC)
- Wouldn't that decompose into O2 + 2O2-? 99.2.148.119 (talk) 00:11, 26 July 2011 (UTC)
- you mean 2 O2 + O22-? Whoop whoop pull up Bitching Betty | Averted crashes 00:30, 26 July 2011 (UTC)
- Either or both. You'd have to put them together to find out, and that might not be possible. If such species were stable, even at cryogenic temperatures, it should be possible to find something about them. If you can find a university chemistry library, they might have something on oxygen that reviews the attempt to synthesize or stabilize the more exotic species. 99.2.148.119 (talk) 01:48, 26 July 2011 (UTC)
- you mean 2 O2 + O22-? Whoop whoop pull up Bitching Betty | Averted crashes 00:30, 26 July 2011 (UTC)
- Wouldn't that decompose into O2 + 2O2-? 99.2.148.119 (talk) 00:11, 26 July 2011 (UTC)
- It would be a chain of six oxygen ions connected by single bonds, with those at the ends carrying one negative charge each (-O-O-O-O-O-O-). Whoop whoop pull up Bitching Betty | Averted crashes 23:11, 25 July 2011 (UTC)
do they really exist? are they stable?--Irrational number (talk) 23:36, 25 July 2011 (UTC)
- I'm not sure they do. Someone would have to work out the molecular orbitals on such species, but I think they would have a bond order of zero, which is pretty much the definition of "doesn't exist". --Jayron32 00:04, 26 July 2011 (UTC)
- I think it should exist if there is a way to make it. Have a look at trioxidane. I would call O2−
4: tetraoxidane-1,4-diide, or if you would like to omit the 1,4 and render them implicit, then just a basic tetraoxidanediide will do. That is, if you prefer substitutive nomenclature, there is no traditional name for this ion, and I don't know the additive name for it. Oh, and O2−
3 is not the ozonide ion, O−
3 is. Plasmic Physics (talk) 00:32, 26 July 2011 (UTC)- Then what is O32-? Whoop whoop pull up Bitching Betty | Averted crashes 00:35, 26 July 2011 (UTC)
- I think it should exist if there is a way to make it. Have a look at trioxidane. I would call O2−
- Trioxidane-1,3-diide or trioxidanediide, it has no traditional name either. Plasmic Physics (talk) 00:48, 26 July 2011 (UTC)
- The trioxidane (HOOOH) article says this compound is also called "hydrogen trioxide", so the parent O2−
3 would be "trioxide" according to the pattern "HOOH is hydrogen peroxide and O2−
2 is the peroxide ion". There are articles that talk about HOOOO• as the "hydrogen tetraoxide radical" and HOOOOH as "hydrogen tetraoxide". DMacks (talk) 01:09, 26 July 2011 (UTC)
- The trioxidane (HOOOH) article says this compound is also called "hydrogen trioxide", so the parent O2−
- Trioxidane-1,3-diide or trioxidanediide, it has no traditional name either. Plasmic Physics (talk) 00:48, 26 July 2011 (UTC)
- The problem is, that they use compositional nomenclature to name these compounds, which does not convey any structural information. I think the additive name for O2−
3 could be trioxygenate(2 O—O)(2-). Plasmic Physics (talk) 01:31, 26 July 2011 (UTC)
- The problem is, that they use compositional nomenclature to name these compounds, which does not convey any structural information. I think the additive name for O2−
- Likewise, O2−
4 could be called: tetraoxygenate(3 O—O)(2-). Plasmic Physics (talk) 02:52, 26 July 2011 (UTC)- Indeed. However, I'm saying what is being used to identify them in the scientific literature:) There certainly are other possible connectivities, especially for higher numbers of oxygen atoms, but it appears that the simple ___oxide refers to the linear one and that other names are used for other structural isomers (just like "hexane" could refer to any C6H14, but it also specifically refers to the linear one). For example, the literature for
H-O(O)-O-H(oxygen-centered analog of sulfurous acid) is identified as "ozonic acid". DMacks (talk) 14:17, 26 July 2011 (UTC)- Correction: ozonic acid is H-O-O(O)-O-H (left out an oxygen in preceding comment). DMacks (talk) 21:01, 26 July 2011 (UTC)
- Indeed. However, I'm saying what is being used to identify them in the scientific literature:) There certainly are other possible connectivities, especially for higher numbers of oxygen atoms, but it appears that the simple ___oxide refers to the linear one and that other names are used for other structural isomers (just like "hexane" could refer to any C6H14, but it also specifically refers to the linear one). For example, the literature for
- Of course, but he was asking the the name of the deprotonated dianion of dihydrogen trioxide. Plasmic Physics (talk) 15:33, 26 July 2011 (UTC)
- I have no idea what you're talking about. I answered each asked case based on literature and actual use (rather than academic follow-the-rules alone--nothing wrong with it, except it's purely academic) and they disprove your concern about compositional naming and instead support the names that do not use the connective rules. My response about 4 was indented as a response to your comment about 4, and my comment about 3 (with extension to 4) was indented as a response to your comment about 3. Even if you aren't using normal indenting schemes, my comments follow each preceding one on the same number. As you broadened the topic (or at least raised new concerns), I responded to each in turn. DMacks (talk) 21:01, 26 July 2011 (UTC)
- Likewise, O2−
- What I meant, is that you are talking about the names for the neutral molecules, when he was asking about the fully deprotonated anions. I just clarified that if you use compositional naming for the neutral molecule, you can't get the name of the anion just by removing terms from the name. How about dicarbon hexachloride, is there such a thing as the hexachloride ion? I don't thinks so! Plasmic Physics (talk) 23:28, 26 July 2011 (UTC)
- Except that terms like 'trioxide' also describe the anion itself. Dihyrdogen trioxide is not merely named because it has three oxygens, it also has the three oxygens which behave as a unit. Hexachloroethane doesn't have that arangement physically. There are other names which could be used analogously to the "trioxide anion", consider the Dihydrogen cation, the Trihydrogen cation, the various names of the Mercury polycations, etc. There is precedence in usage for these terms. --Jayron32 02:16, 27 July 2011 (UTC)
- What I meant, is that you are talking about the names for the neutral molecules, when he was asking about the fully deprotonated anions. I just clarified that if you use compositional naming for the neutral molecule, you can't get the name of the anion just by removing terms from the name. How about dicarbon hexachloride, is there such a thing as the hexachloride ion? I don't thinks so! Plasmic Physics (talk) 23:28, 26 July 2011 (UTC)
Organic ozonides
If organic peroxides such as acetone peroxide are explosives, then are the even-more-reactive organic ozonides such as molozonide explosive? Whoop whoop pull up Bitching Betty | Averted crashes 22:26, 25 July 2011 (UTC)
- It probably depends a LOT on the identity of the "R" groups. Usually, molozonides are not isolatable products, but rather exist as metastable intermediates as part of larger mechanisms, for example ozonolysis. That is, you don't ever have a bottle of molozonides sitting on a shelf; they exist primarily in miniscule trace amounts during larger synthetic mechanisms. --Jayron32 00:01, 26 July 2011 (UTC)
- If you could somehow suddenly isolate a bottlefull of pure molozonides, would it decompose explosively? Whoop whoop pull up Bitching Betty | Averted crashes 00:37, 26 July 2011 (UTC)
- By what mechanism would you isolate it? --Jayron32 00:41, 26 July 2011 (UTC)
- I dunno, what I meant is, if somehow a bottleful of pure molozonides came into existance, would it decompose explosively? Whoop whoop pull up Bitching Betty | Averted crashes 00:43, 26 July 2011 (UTC)
- So you've invented a world full of magic whereby bottles of compounds can "pop" into existance without mechanism or cause? Its your magic world, perhaps you can decide whether it would be explosive or not... --Jayron32 00:46, 26 July 2011 (UTC)
- Nononononononononono. I meant, does their decomposition have enough power per molecule that it would be explosive if it were ramped up to the scale of a bottle-ful? Whoop whoop pull up Bitching Betty | Averted crashes 00:49, 26 July 2011 (UTC)
- I'll stop being flippant about this. The deal is, if the substance has not been isolated, there exists no empirical evidence as to its properties in this regard. The amount of energy evolved in decomposition is not the sole determining factor in deciding if it explodes. Indeed, the actual amount of that energy can be trivially approximated using basic thermodynamics. However, that number means nothing if the energy is released slowly. You would need to know kinetic information about the decomposition reaction, that is how fast it decomposes. After all, thermodynamically the reaction we call rusting is fundementally, in nearly every way, identical to the reaction we call burning. The difference is that rusting is very slow, while burning is very fast. The same deal here; deciding how much it will explode would actually require a kinetic study of the mechanism, and you would actually need to isolate the compound to do that study. --Jayron32 01:02, 26 July 2011 (UTC)
- Are there any isolatable organic ozonides that could be used as explosives? Whoop whoop pull up Bitching Betty | Averted crashes 13:39, 26 July 2011 (UTC)
- Probably not. Per this abstract, ozonides have a "finite lifetime", i.e. they cannot be isolated and stored. In other words, though they form as real intermediates during the process of ozonolysis, you can't "stop" the process and exctract them from the reaction system. --Jayron32 16:26, 26 July 2011 (UTC)
- Okay, so the primary ozonides (1,2,3,-trioxolanes) are nonisolatable. What about the so-called secondary ozonides (1,2,4,-trioxolanes?) Whoop whoop pull up Bitching Betty | Averted crashes 21:16, 26 July 2011 (UTC)
- doi:10.1002/ciuz.19730070303 and doi:10.1002/hlca.200490186 do not point out that they are very explosive.--Stone (talk) 21:28, 26 July 2011 (UTC)
- Okay, so the primary ozonides (1,2,3,-trioxolanes) are nonisolatable. What about the so-called secondary ozonides (1,2,4,-trioxolanes?) Whoop whoop pull up Bitching Betty | Averted crashes 21:16, 26 July 2011 (UTC)
- Probably not. Per this abstract, ozonides have a "finite lifetime", i.e. they cannot be isolated and stored. In other words, though they form as real intermediates during the process of ozonolysis, you can't "stop" the process and exctract them from the reaction system. --Jayron32 16:26, 26 July 2011 (UTC)
- Are there any isolatable organic ozonides that could be used as explosives? Whoop whoop pull up Bitching Betty | Averted crashes 13:39, 26 July 2011 (UTC)
- I'll stop being flippant about this. The deal is, if the substance has not been isolated, there exists no empirical evidence as to its properties in this regard. The amount of energy evolved in decomposition is not the sole determining factor in deciding if it explodes. Indeed, the actual amount of that energy can be trivially approximated using basic thermodynamics. However, that number means nothing if the energy is released slowly. You would need to know kinetic information about the decomposition reaction, that is how fast it decomposes. After all, thermodynamically the reaction we call rusting is fundementally, in nearly every way, identical to the reaction we call burning. The difference is that rusting is very slow, while burning is very fast. The same deal here; deciding how much it will explode would actually require a kinetic study of the mechanism, and you would actually need to isolate the compound to do that study. --Jayron32 01:02, 26 July 2011 (UTC)
- Nononononononononono. I meant, does their decomposition have enough power per molecule that it would be explosive if it were ramped up to the scale of a bottle-ful? Whoop whoop pull up Bitching Betty | Averted crashes 00:49, 26 July 2011 (UTC)
- So you've invented a world full of magic whereby bottles of compounds can "pop" into existance without mechanism or cause? Its your magic world, perhaps you can decide whether it would be explosive or not... --Jayron32 00:46, 26 July 2011 (UTC)
- I dunno, what I meant is, if somehow a bottleful of pure molozonides came into existance, would it decompose explosively? Whoop whoop pull up Bitching Betty | Averted crashes 00:43, 26 July 2011 (UTC)
- By what mechanism would you isolate it? --Jayron32 00:41, 26 July 2011 (UTC)
- If you could somehow suddenly isolate a bottlefull of pure molozonides, would it decompose explosively? Whoop whoop pull up Bitching Betty | Averted crashes 00:37, 26 July 2011 (UTC)
But others do (doi:10.1016/j.tet.2006.08.092 and Org Syn Coll 7 p168). There have been some industrial and academic accidents resulting from incomplete "whatever the next reaction is" when that product is isolated. However, some with large substituents are extremely stable. Things that look like they came from cyclohexylidine-cyclohexane and beyond are well studied and even have some biochemical/pharmacological properties. Interestingly the ones that are stable are often not formed by ozonolysis of the seeming parent alkene. DMacks (talk) 21:47, 26 July 2011 (UTC)
- That actually makes perfect sense. Peroxides and ozonides are essentially both highly electronegative AND highly electron deficient, which is why they are so unstable, so the presence of electron-donating groups like large, bulky alkyl groups should have a stabilizing effect on their decomoposition. --Jayron32 02:05, 27 July 2011 (UTC)
