Alkene: Difference between revisions
| Line 205: | Line 205: | ||
A single ketone can also be converted to the corresponding alkene via its tosylhydrazone, using [[sodium methoxide]] (the [[Bamford-Stevens reaction]]) or an alkyllithium (the [[Shapiro reaction]]). |
A single ketone can also be converted to the corresponding alkene via its tosylhydrazone, using [[sodium methoxide]] (the [[Bamford-Stevens reaction]]) or an alkyllithium (the [[Shapiro reaction]]). |
||
===Olefin metathesis=== |
===Synthesis from alkenes: Olefin metathesis and Hydrovinylation=== |
||
{{main|Olefin metathesis}} |
{{main|Olefin metathesis}} |
||
Alkenes can be prepared by exchange with other alkenes, in a reaction known as [[olefin metathesis]]. Frequently, loss of ethene gas is used to drive the reaction towards a desired product. In many cases, a mixture of geometric isomers is obtained, but the reaction tolerates many functional groups. The method is particularly effective for the preparation of cyclic alkenes, as in this synthesis of [[muscone]]: |
Alkenes can be prepared by exchange with other alkenes, in a reaction known as [[olefin metathesis]]. Frequently, loss of ethene gas is used to drive the reaction towards a desired product. In many cases, a mixture of geometric isomers is obtained, but the reaction tolerates many functional groups. The method is particularly effective for the preparation of cyclic alkenes, as in this synthesis of [[muscone]]: |
||
[[Image:MusconeViaRCM.png|500px|center|Ring-closing metathesis used in synthesis of muscone]] |
[[Image:MusconeViaRCM.png|500px|center|Ring-closing metathesis used in synthesis of muscone]] |
||
Transition metal catalyzed hydrovinylation of alkenes is also an important alkene synthesis process. In general, it involves the addition of a hydrogen and a vinyl (or an alkenyl group) across a double bond. The hydrovinylation reaction was first reported by Alderson et al. by using rhodium and ruthenium salts, other metal catalysts commonly employed nowadays included iron, cobalt, nickel, and palladium. The addition can be done highly regio- and stereo-selectively, the choices of ligands, substrates and counterions often play very important role. |
|||
===Coupling reactions=== |
===Coupling reactions=== |
||
Revision as of 07:02, 29 September 2010

In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond.[1] The simplest acyclic alkenes, with only one double bond and no other functional groups, form an homologous series of hydrocarbons with the general formula CnH2n.[2]
The simplest alkene is ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene. Alkenes are also called olefins (an archaic synonym, widely used in the petrochemical industry). Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.[2]
Structure
Bonding

Like single covalent bonds, double bonds can be described in terms of overlapping atomic orbitals, except that, unlike a single bond (which consists of a single sigma bond), a carbon-carbon double bond consists of one sigma bond and one pi bond. This double bond is stronger than a single covalent bond (611 kJ/mol for C=C vs. 347 kJ/mol for C—C) [1] and also shorter with an average bond length of 1.33 Angstroms (133 pm).
Each carbon of the double bond uses its three sp² hybrid orbitals to form sigma bonds to three atoms. The unhybridized 2p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp² hybrid orbitals, combine to form the pi bond. This bond lies outside the main C—C axis, with half of the bond on one side and half on the other.
Rotation about the carbon-carbon double bond is restricted because it involves breaking the pi bond, which requires a large amount of energy (264 kJ/mol in ethylene). As a consequence, substituted alkenes may exist as one of two isomers, called cis or trans isomers. More complex alkenes may be named using the E-Z notation, used to describe molecules having three or four different substituents (side groups). For example, of the isomers of butene, the two methyl groups of (Z)-but-2-ene (aka cis-2-butene) face the same side of the double bond, and in (E)-but2-ene (aka trans-2-butene) the methyl groups face the opposite side. These two isomers of butene are slightly different in their chemical and physical properties.
It is certainly not impossible to twist a double bond. In fact, a 90° twist requires an energy approximately equal to half the strength of a pi bond. The misalignment of the p orbitals is less than expected because pyramidalization takes place (See: pyramidal alkene). trans-Cyclooctene is a stable strained alkene and the orbital misalignment is only 19° with a dihedral angle of 137° (normal 120°) and a degree of pyramidalization of 18°. This explains the dipole moment of 0.8 D for this compound (cis-isomer 0.4 D) where a value of zero is expected.[3] The trans isomer of cycloheptene is only stable at low temperatures.
Shape
As predicted by the VSEPR model of electron pair repulsion, the molecular geometry of alkenes includes bond angles about each carbon in a double bond of about 120°. The angle may vary because of steric strain introduced by nonbonded interactions created by functional groups attached to the carbons of the double bond. For example, the C-C-C bond angle in propylene is 123.9°.
Physical properties
The physical properties of alkenes are comparable with those of alkanes. The physical state depends on molecular mass (gases from ethene to butene - liquids from pentene onwards). The simplest alkenes, ethene, propene and butene are gases. Linear alkenes of approximately five to sixteen carbons are liquids, and higher alkenes are waxy solids.
Reactions
Alkenes are relatively stable compounds, but are more reactive than alkanes due to the presence of a carbon-carbon pi-bond. It is also attributed to the presence of pi-electrons in the molecule. The majority of the reactions of alkenes involve the rupture of this pi bond, forming new single bonds.
Alkenes serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions.
Addition reactions
Alkenes react in many addition reactions, which occur by opening up the double-bond. Most addition reactions to alkenes follow the mechanism of electrophilic addition
Hydrogenation
Hydrogenation of alkenes produces the corresponding alkanes. The reaction is carried out under pressure at a temperature of 200°c in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickel or palladium. For laboratory syntheses, Raney nickel (an alloy of nickel and aluminium) is often employed. The simplest example of this reaction is the catalytic hydrogenation of ethylene to yield ethane:
- CH2=CH2 + H2 → CH3-CH3
Halogenation
In electrophilic halogenation the addition of elemental bromine or chlorine to alkenes yields vicinal dibromo- and dichloroalkanes, respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:
- CH2=CH2 + Br2 → BrCH2-CH2Br
It is also used as a quantitive test of unsaturation, expressed as the bromine number of a single compound or mixture. The reaction works because the high electron density at the double bond causes a temporary shift of electrons in the Br-Br bond causing a temporary induced dipole. This makes the Br closest to the double bond slightly positive and therefore an electrophile.
Hydrohalogenation is the addition of hydrohalic acids such as HCl or HBr to alkenes to yield the corresponding haloalkanes.
- CH3-CH=CH2 + HBr → CH3-CHBr-CH2-H
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents (Markovnikov's rule).
Oxidation
Alkenes are oxidized with a large number of oxidizing agents. In the presence of oxygen, alkenes burn with a bright flame to produce carbon dioxide and water. Catalytic oxidation with oxygen or the reaction with percarboxylic acids yields epoxides. Reaction with ozone in ozonolysis leads to the breaking of the double bond, yielding two aldehydes or ketones. Reaction with Concentrated, Hot KMnO4 (or other oxidizing salts) in an acidic solution will yield ketones or carboxylic acids.
- R1-CH=CH-R2 + O3 → R1-CHO + R2-CHO + H2O
This reaction can be used to determine the position of a double bond in an unknown alkene.
Polymerization
Polymerization of alkenes is a reaction that yields polymers of high industrial value at great economy, such as the plastics polyethylene and polypropylene. Polymers from alkene monomers are referred to in a general way as polyolefins or in rare instances as polyalkenes. To be more specific, a polymer from alpha-olefins is called a polyalphaolefin (PAO). Polymerization can proceed via either a free-radical or an ionic mechanism, converting the double to a single bond and forming single bonds to join the other monomers. Polymerization of conjugated dienes such as buta-1,3-diene or isoprene (2-methylbuta-1,3-diene) results in largely 1,4-addition with possibly some 1,2-addition of the diene monomer to a growing polymer chain. For details, see "Polybutadiene".
Reaction overview
| Reaction name | Product | Comment |
|---|---|---|
| Hydrogenation | alkanes | addition of hydrogen |
| Hydroalkenylation | alkenes | hydrometalation / insertion / beta elimination by metal catalyst |
| Halogen addition reaction | 1,2-dihalide | electrophilic addition of halogens |
| Hydrohalogenation | haloalkanes | addition of hydrohalic acids |
| Hydroamination | amines | addition of N-H bond across C-C double bond |
| Hydroformylation | aldehydes | industrial process, addition of CO and H2 |
| Sharpless bishydroxylation | diols | oxidation, reagent: osmium tetroxide , chiral ligand |
| Woodward cis-hydroxylation | diols | oxidation, reagents: iodine, silver acetate |
| ozonolysis | aldehydes or ketones | reagent: ozone |
| Olefin metathesis | alkenes | two alkenes rearrange to form two new alkenes |
| Diels-Alder reaction | cyclohexenes | cycloaddition with a diene |
| Pauson-Khand reaction | cyclopentenones | cycloaddition with an alkyne and CO |
| Hydroboration–oxidation | alcohols | reagents: borane , then a peroxide |
| oxymercuration-reduction | alcohols | electrophilic addition of mercuric acetate, then reduction |
| Prins reaction | 1,3-diols | electrophilic addition with aldehyde or ketone |
| Paterno–Büchi reaction | oxetanes | photochemical reaction with aldehyde or ketone |
| Epoxidation | epoxide | electrophilic addition of an peroxide |
| Cyclopropanation | cyclopropanes | addition of carbenes or carbenoids |
| Hydroacylation | ketones | oxidative addition / reductive elimination by metal catalyst |
Synthesis
Industrial methods
The most common industrial synthesis of alkenes is based on cracking of petroleum. Large alkanes are broken apart at high temperatures, often in the presence of a zeolite catalyst, to give alkenes and smaller alkanes, and the mixture of products is then separated by fractional distillation. This is mainly used for the manufacture of small alkenes (up to six carbons).[1]

Related to this is catalytic dehydrogenation, where an alkane loses hydrogen at high temperatures to produce a corresponding alkene. [1] This is the reverse of the catalytic hydrogenation of alkenes.
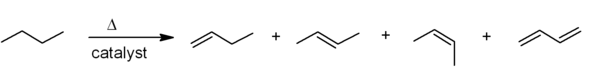
Both of these processes are endothermic, but they are driven towards the alkene at high temperatures by entropy (the TΔS portion of the equation ΔG = ΔH – TΔS dominates for high T).
Catalytic synthesis of higher α-alkenes (of the type RCH=CH2) can also be achieved by a reaction of ethylene with the organometallic compound triethylaluminium in the presence of nickel, cobalt, or platinum.
Elimination reactions
One of the principal methods for alkene synthesis in the laboratory is the elimination of alkyl halides, alcohols, and similar compounds. Most common is the β-elimination via the E2 or E1 mechanism,[4] but α-eliminations are also known.
The E2 mechanism provides a more reliable β-elimination method than E1 for most alkene syntheses. Most E2 eliminations start with an alkyl halide or alkyl sulfonate ester (such as a tosylate or triflate). When an alkyl halide is used, the reaction is called a dehydrohalogenation. For unsymmetrical products, the more substituted alkenes (those with fewer hydrogens attached to the C=C) tend to predominate (see Saytzeff's rule). Two common methods of elimination reactions are dehydrohalogenation of alkyl halides and dehydration of alcohols. A typical example is shown below; note that the H that leaves must be anti to the leaving group, even though this leads to the less stable Z-isomer.[5]
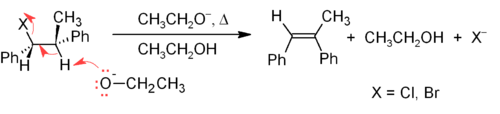
Alkenes can be synthesized from alcohols via dehydration, in which case water is lost via the E1 mechanism. For example, the dehydration of ethanol produces ethene:
An alcohol may also be converted to a better leaving group (e.g., xanthate), so as to allow a milder syn-elimination such as the Chugaev elimination and the Grieco elimination. Related reactions include eliminations by β-haloethers (the Boord olefin synthesis) and esters (ester pyrolysis).
Alkenes can be prepared indirectly from alkyl amines. The amine or ammonia is not a suitable leaving group, so the amine is first either alkylated (as in the Hofmann elimination) or oxidized to an amine oxide (the Cope reaction) to render a smooth elimination possible. Hofmann elimination is unusual in that the less substituted (non-Saytseff) alkene is usually the major product. The Cope reaction is a syn-elimination that occurs at or below 150 °C, for example:[6]
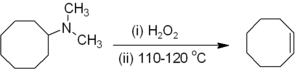
Alkenes are generated from α-halo sulfones in the Ramberg-Bäcklund reaction, via a three-membered ring sulfone intermediate.
Synthesis from carbonyl compounds
Another important method for alkene synthesis involves construction of a new carbon-carbon double bond by coupling of a carbonyl compound (such as an aldehyde or ketone) to a carbanion equivalent. Such reactions are sometimes called olefinations. The most well-known of these methods is the Wittig reaction, but other related methods are known.
The Wittig reaction involves reaction of an aldehyde or ketone with a Wittig reagent (or phosphorane) of the type Ph3P=CHR to produce an alkene and Ph3P=O. The Wittig reagent is itself prepared easily from triphenylphosphine and an alkyl halide. The reaction is quite general and many functional groups are tolerated, even esters, as in this example:[7]

Related to the Wittig reaction is the Peterson olefination. This uses a less accessible silicon-based reagent in place of the phosphorane, but it allows for the selection of E or Z products. If an E-product is desired, another alternative is the Julia olefination, which uses the carbanion generated from a phenyl sulfone. The Takai olefination based on an organochromium intermediate also delivers E-products. A titanium compound, Tebbe's reagent, is useful for the synthesis of methylene compounds; in this case, even esters and amides react.
A pair of carbonyl compounds can also be reductively coupled together (with reduction) to generate an alkene. Symmetrical alkenes can be prepared from a single aldehyde or ketone coupling with itself, using Ti metal reduction (the McMurry reaction). If two different ketones are to be coupled, a more complex, indirect method such as the Barton-Kellogg reaction may be used.
A single ketone can also be converted to the corresponding alkene via its tosylhydrazone, using sodium methoxide (the Bamford-Stevens reaction) or an alkyllithium (the Shapiro reaction).
Synthesis from alkenes: Olefin metathesis and Hydrovinylation
Alkenes can be prepared by exchange with other alkenes, in a reaction known as olefin metathesis. Frequently, loss of ethene gas is used to drive the reaction towards a desired product. In many cases, a mixture of geometric isomers is obtained, but the reaction tolerates many functional groups. The method is particularly effective for the preparation of cyclic alkenes, as in this synthesis of muscone:

Transition metal catalyzed hydrovinylation of alkenes is also an important alkene synthesis process. In general, it involves the addition of a hydrogen and a vinyl (or an alkenyl group) across a double bond. The hydrovinylation reaction was first reported by Alderson et al. by using rhodium and ruthenium salts, other metal catalysts commonly employed nowadays included iron, cobalt, nickel, and palladium. The addition can be done highly regio- and stereo-selectively, the choices of ligands, substrates and counterions often play very important role.
Coupling reactions
Coupling reactions, most notably those catalyzed by palladium compounds, have become popular for the synthesis of alkenes.[8] The Heck reaction provides a method for coupling an aryl halide to an alkene, for example in the synthesis of the pharmaceutical naproxen:
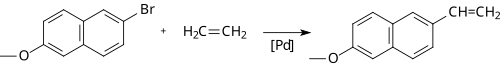
Other couplings, such as the Stille, Suzuki and Negishi involve the reaction of an alkenyl, allyl or aryl halide (or triflate) with an alkenyl, alkyl (not for Stille) or aryl derivative of a metal or metalloid. For example, Suzuki coupling has been used on a citronellal derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:[9]
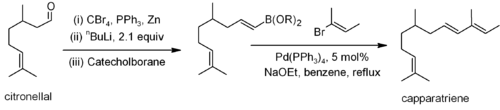
From alkynes
Reduction of alkynes is a useful method for the stereoselective synthesis of disubstituted alkenes. If the cis-alkene is desired, hydrogenation in the presence of Lindlar's catalyst is commonly used, though hydroboration followed by hydrolysis provides an alternative approach. Reduction of the alkyne by sodium metal in liquid ammonia gives the trans-alkene.[10]
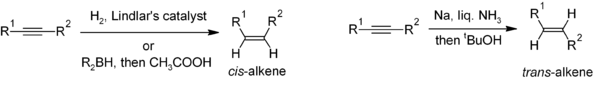
For the preparation multisubstituted alkenes, carbometalation of alkynes can give rise to a large variety of alkene derivatives.
Rearrangements and related reactions
Alkenes can be synthesized from other alkenes via rearrangement reactions. Besides olefin metathesis (described above), a large number of pericyclic reactions can be used such as the ene reaction and the Cope rearrangement.
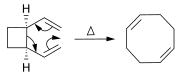
In the Diels-Alder reaction, a cyclohexene derivative is prepared from a diene and a reactive or electron-deficient alkene.
Nomenclature
IUPAC Names
To form the root of the IUPAC names for alkenes, simply change the -an- infix of the parent to -en-. For example, CH3-CH3 is the alkane ethANe. The name of CH2=CH2 is therefore ethENe.
In higher alkenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest carbon chain that contains the double bond in the direction that gives the carbon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first carbon.
- Name branched or substituted alkenes in a manner similar to alkanes.
- Number the carbon atoms, locate and name substituent groups, locate the double bond, and name the main chain.

Cis-Trans notation
In the specific case of disubstituted alkenes where the two carbons have one substituent each, Cis-trans notation may be used. If both substituents are on the same side of the bond, it is defined as (cis-). If the substituents are on either side of the bond, it is defined as (trans-).

E,Z notation
When an alkene has more than one substituent (especially necessary with 3 or 4 substituents), the double bond geometry is described using the labels E and Z. These labels come from the German words "entgegen," meaning "opposite," and "zusammen," meaning "together." Alkenes with the higher priority groups (as determined by CIP rules) on the same side of the double bond have these groups together and are designated Z. Alkenes with the higher priority groups on opposite sides are designated E. A mnemonic to remember this: Z notation has the higher priority groups on "ze zame zide."

Groups containing C=C double bonds
IUPAC recognizes two names for hydrocarbon groups containing carbon-carbon double bonds, the vinyl group and the allyl group. .[2]
See also
References
- ^ a b c d Wade, L.G. (Sixth Ed., 2006). Organic Chemistry. Pearson Prentice Hall. p. 279. ISBN 140585345X.
{{cite book}}: Check date values in:|date=(help) Cite error: The named reference "Wade" was defined multiple times with different content (see the help page). - ^ a b c Moss, G. P.; Smith, P. A. S.; Tavernier, D. (1995). "Glossary of Class Names of Organic Compounds and Reactive Intermediates Based on Structure (IUPAC Recommendations 1995)". Pure and Applied Chemistry. 67: 1307–1375. doi:10.1351/pac199567081307. Cite error: The named reference "PAC1995" was defined multiple times with different content (see the help page).
- ^ Barrows, Susan E.; Eberlein, Thomas H. (2005). "Understanding Rotation about a C=C Double Bond". J. Chem. Educ. 82: 1329. doi:10.1021/ed082p1329.
- ^ Saunders, W. H. (1964). Patai, Saul (ed.). The Chemistry of Alkenes. Wiley Interscience. pp. 149–150.
- ^ Cram, D.J.; Greene, Frederick D.; Depuy, C. H. (1956). "Studies in Stereochemistry. XXV. Eclipsing Effects in the E2 Reaction1". Journal of the American Chemical Society. 78 (4): 790–796. doi:10.1021/ja01585a024.
{{cite journal}}:|access-date=requires|url=(help) - ^ Bach, R.D.; Andrzejewski, Denis; Dusold, Laurence R. (1973). "Mechanism of the Cope elimination". J. Org. Chem. 38: 1742–3. doi:10.1021/jo00949a029.
- ^ Snider, Barry B.; Matsuo, Y; Snider, BB (2006). "Synthesis of ent-Thallusin". Org. Lett. 8 (10): 2123–6. doi:10.1021/ol0605777. PMC 2518398. PMID 16671797.
- ^ Zweifel, George S. (2007). Modern Organic Synthesis: An Introduction. New York: W. H. Freeman & Co. pp. 322–339. ISBN 0716772663.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vyvyan, J.R. (1999). "An expedient total synthesis of (+/-)-caparratriene". Tetrahedron Letters. 40 (27): 4947–4949. doi:10.1016/S0040-4039(99)00865-5. Retrieved 2008-01-02.
- ^ Zweifel, George S. (2007). Modern Organic Synthesis: An Introduction. New York: W. H. Freeman & Co. p. 366. ISBN 0716772663.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)


