Water: Difference between revisions
rv minor vandalism to last edit by SS |
restructure according to WikiProject Science; drop section on cloth |
||
| Line 78: | Line 78: | ||
|} |
|} |
||
Water is a substance found almost everywhere on [[earth]] and is required by all known [[life]]. About 70% of the Earth's surface is covered by water. Water is known to exist, in [[ice]] form, on several other bodies in the [[solar system]] and beyond. The importance of water to terrestrial life has led many to surmise that its existence in liquid form anywhere besides Earth could provide a viable habitat for [[extraterrestrial]] life. |
|||
'''Water''' is [[liquid]] at [[standard temperature and pressure]]. Its other common forms are [[ice]], [[snow]] and [[water vapor|vapor]]. It has the [[chemical formula]] [[hydrogen|H]]<sub>2</sub>[[oxygen|O]], meaning that one [[molecule]] of water is composed of two [[hydrogen]] [[atom]]s and one [[oxygen]] atom. |
|||
==General== |
|||
The [[solid state]] of water is known as [[ice]]; the [[gaseous state]] is known as [[water vapor]] (or [[steam]]). The units of temperature (formerly the degree [[Celsius]] and now the [[Kelvin]]) are defined in terms of the [[triple point]] of water, 273.16 K (0.01 °C) and 611.2 Pa, the temperature and pressure at which solid, liquid, and [[Water vapor|gaseous water]] coexist in equilibrium. Water exhibits some very strange behaviors, including the formation of states such as [[vitreous ice]], a noncrystalline (glassy), solid state of water. |
|||
==Water in practice== |
|||
At temperatures greater than 647 [[Kelvin|K]] and pressures greater than 22.064 [[pascal|MPa]], a collection of water molecules assumes a ''supercritical'' condition, in which liquid-like clusters float within a vapor-like phase. |
|||
===Forms of water=== |
|||
The [[liquid water path]] is a measure of the amount of liquid water in an air column. |
|||
:''See the category: [[:Category:Forms of water]]'' |
|||
The [[solid state]] of water is known as [[ice]]; the [[gaseous state]] is known as [[water vapor]] (or [[steam]]). |
|||
Water exhibits some very strange behaviors, including the formation of states such as [[vitreous ice]], a noncrystalline (glassy), solid state of water. |
|||
==Body of water== |
|||
A '''body of water''' is an [[ocean]], [[sea]], [[lake]], [[river]], [[stream]], [[canal]], [[pond]], or the like. See [[water resources]] for information about fresh water supplies. Also see: [[sea water]], [[fresh water]], and [[underwater]]. |
|||
At temperatures greater than 647 [[Kelvin|K]] and pressures greater than 22.064 [[pascal|MPa]], a collection of water molecules assumes a ''supercritical'' condition, in which liquid-like clusters float within a vapor-like phase. |
|||
Earth's approximate water volume (the total water supply of the world) is 1,360,000,000 km³ (326,000,000 mile³). Of this volume: |
|||
===Water properties=== |
|||
* 1,320,000,000 km³ (316,900,000 mile³ or 97.2%) is in the oceans |
|||
Just like most other materials, liquid water becomes denser with lowering temperature. However, unlike most other materials, when cooled to near freezing point, it becomes '''lighter''' with lowering temperature. Liquid water reaches its highest density at a temperature of 4 °C. |
|||
* 25,000,000 km³ (6,000,000 mile³ or 1.8%) is in glaciers and icecaps |
|||
* 13,000,000 km³ (3,000,000 mile³ or 0.9%) is groundwater. |
|||
* 250,000 km³ (60,000 mile³ or 0.02%) is fresh water in lakes, inland seas, and rivers. |
|||
* 13,000 km³ (3,100 mile³ or 0.001%) is atmospheric water vapour at any given time. |
|||
This has an interesting consequence for water life in winter. Water chilled at the surface becomes denser and sinks, forming convection currents that cool the whole water body, but when the temperature of the lake water reaches 4°C, water on the surface, as it chills further, becomes ''less dense'', and stays as a surface layer which eventually forms ice. Since downward convection of colder water is blocked by the density change, any large body of water frozen in winter will have the bulk of its water still liquid at 4°C beneath the icy surface, allowing fish to survive. This is one of the principal examples of finely-tuned physical properties that support life on Earth that is used as an argument for the [[anthropic principle]]. |
|||
==The dipolar nature of water== |
|||
An important feature of water is its [[polar molecule|polar]] nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher [[electronegativity]] than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a [[dipole]]. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as [[hydrogen bond]]ing. |
|||
The [[temperature]] and [[pressure]] at which solid, liquid, and [[Water vapor|gaseous water]] coexist in equilibrium is called the [[triple point]] of water. This point is used to define the units of temperature (formerly the degree [[Celsius]] and now the [[Kelvin]]). The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.2 [[Pascal|Pa]]. |
|||
This relatively weak (relative to the covalent bonds within the water molecule itself) attraction results in physical properties such as a relatively high [[boiling point]], because a lot of [[heat]] energy is necessary to break the hydrogen bonds between molecules. For example, [[sulfur]] is the element below oxygen in the periodic table, and its equivalent compound, hydrogen sulfide (H<sub>2</sub>S) does not have hydrogen bonds, and though it has twice the molecular weight of water, it is a gas at [[room temperature]]. The extra bonding between water molecules also gives liquid water a large [[specific heat capacity]]. |
|||
Water is also a good [[solvent]]. The solvent properties of water are vital in [[biology]], because many biochemical reactions take place only within aqueous [[solution]]s (e.g., reactions in the [[cytoplasm]] and [[blood]]). In addition, water is used to transport [[biological molecule]]s. |
|||
Hydrogen bonding also gives water an unusual behaviour when freezing. Just like most other materials, the liquid becomes denser with lowering temperature. However, unlike most other materials, when cooled to near freezing point, the presence of hydrogen bonds means that the molecules, as they rearrange to minimise their energy, form a structure that is actually of lower density: hence the solid form, ice, will float in water. In other words, water expands as it freezes (most other materials shrink on solidification). Liquid water reaches its highest density at a temperature of 4 °C. This has an interesting consequence for water life in winter. Water chilled at the surface becomes denser and sinks, forming convection currents that cool the whole water body, but when the temperature of the lake water reaches 4°C, water on the surface, as it chills further, becomes ''less dense'', and stays as a surface layer which eventually forms ice. Since downward convection of colder water is blocked by the density change, any large body of water frozen in winter will have the bulk of its water still liquid at 4°C beneath the icy surface, allowing fish to survive. This is one of the principal examples of finely-tuned physical properties that support life on Earth that is used as an argument for the [[anthropic principle]]. |
|||
Water drops are stable thanks to the high [[surface tension]] of water. This can be seen when small quantities of water are put onto a nonsoluble surface: the water stays together as drops. This property is important for life, e.g. when water is carried through [[xylem]] up stems in plants : the strong intermolecular attractions hold the water column together, and prevent tension caused by [[transpiration pull]]. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem vessel inoperative. |
|||
Another consequence is that ice will melt if sufficient pressure is applied. |
|||
==Water as a solvent== |
|||
Water is also a good [[solvent]] due to its polarity. The solvent properties of water are vital in [[biology]], because many biochemical reactions take place only within aqueous [[solution]]s (e.g., reactions in the [[cytoplasm]] and [[blood]]). In addition, water is used to transport [[biological molecule]]s. |
|||
When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small size of water molecules typically allows many water molecules to surround one molecule of ''solute''. The partially negative dipoles of the water are attracted to positively charged components of the solute, and vice versa for the positive dipoles. |
|||
In general, ionic and polar substances such as [[acid]]s, [[alcohol]]s, and [[salt]]s are easily soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in [[van der Waals force|van der Waals interactions]] with nonpolar molecules. |
|||
An example of an ionic solute is [[sodium chloride|table salt]]; the sodium chloride, NaCl, separates into Na<sup>+</sup> [[cation]]s and Cl<sup>-</sup> [[anion]]s, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is [[sucrose|table sugar]]. The water dipoles hydrogen bond to the dipolar regions of the sugar molecule and allow it to be carried away into solution. |
|||
==Cohesion and surface tension== |
|||
The strong hydrogen bonds give water a high cohesiveness and, consequently, [[surface tension]]. This is evident when small quantities of water are put onto a nonsoluble surface and the water stays together as drops. This feature is important when water is carried through [[xylem]] up stems in plants; the strong intermolecular attractions hold the water column together, and prevent tension caused by [[transpiration pull]]. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem vessel inoperative. |
|||
==Conductivity== |
|||
''Pure'' water is actually a good [[insulator]] (poor [[conductor]]), meaning that it does ''not'' conduct [[electricity]] well. Because water is such a good solvent, however, it often has some [[solvent|solute]] dissolved in it, most frequently salt. If water has such impurities, then it can conduct electricity much better, because impurities such as salt comprise free [[ions]] in aqueous solution by which an electric current can flow. |
''Pure'' water is actually a good [[insulator]] (poor [[conductor]]), meaning that it does ''not'' conduct [[electricity]] well. Because water is such a good solvent, however, it often has some [[solvent|solute]] dissolved in it, most frequently salt. If water has such impurities, then it can conduct electricity much better, because impurities such as salt comprise free [[ions]] in aqueous solution by which an electric current can flow. |
||
Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called [[electrolysis]]. Water molecules naturally disassociate into H<sup>+</sup> and OH<sup>-</sup> ions, which are pulled toward the [[cathode]] and [[anode]], respectively. At the cathode, two H<sup>+</sup> ions pick up electrons and form H<sub>2</sub> gas. At the anode, four OH<sup>-</sup> ions combine and release O<sub>2</sub> gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. |
|||
==Electrolysis== |
|||
Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called ''electrolysis''. |
|||
Water molecules naturally disassociate into H<sup>+</sup> and OH<sup>-</sup> ions, which are pulled toward the [[cathode]] and [[anode]], respectively. |
|||
At the cathode, two H<sup>+</sup> ions pick up electrons and form H<sub>2</sub> gas. At the anode, four OH<sup>-</sup> ions combine and release O<sub>2</sub> gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. |
|||
In theory, pure water has a [[pH]] of 7. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will rapidly dissolve carbon dioxide, forming a solution of carbonic acid, with a limiting pH of ~5.7. |
|||
==Reactivity== |
|||
===Water on Earth=== |
|||
Chemically, water is [[amphoteric]]: able to act as an acid or base. Occasionally the term ''hydroxic acid'' is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of [[hydroxide]] ions (OH<sup>-</sup>) is equal to that of the [[hydronium]] (H<sub>3</sub>O<sup>+</sup>) or [[hydrogen]] ions (H<sup>+</sup>) ions. If the [[equilibrium]] is disturbed, the solution becomes acidic (higher concentration of hydronium ions) or basic (higher concentration of hydroxide ions). |
|||
Liquid water is found in '''body of water''', such as an [[ocean]], [[sea]], [[lake]], [[river]], [[stream]], [[canal]], [[pond]], or the like. See [[water resources]] for information about fresh water supplies. Also see: [[sea water]], [[fresh water]], and [[underwater]]. |
|||
Earth's approximate water volume (the total water supply of the world) is 1,360,000,000 km³ (326,000,000 mile³). Of this volume: |
|||
Water can act as either an acid or an alkali in reactions. According to the [[Brønsted-Lowry]] system, an acid defined as a species which donates a proton (a H<sup>+</sup> ion) in a reaction, and an alkali as something which receives a proton. When reacting with a stronger acid, water acts as an alkali, and will act as an acid when reaction with a weaker acid. For instance, it will receive a H+ from HCl in the equilibrium: |
|||
* 1,320,000,000 km³ (316,900,000 mile³ or 97.2%) is in the oceans |
|||
:HCl + H<sub>2</sub>O → H<sub>3<sub>O<sup>+</sup> + Cl<sup>-</sup> |
|||
* 25,000,000 km³ (6,000,000 mile³ or 1.8%) is in glaciers and icecaps |
|||
* 13,000,000 km³ (3,000,000 mile³ or 0.9%) is groundwater. |
|||
* 250,000 km³ (60,000 mile³ or 0.02%) is fresh water in lakes, inland seas, and rivers. |
|||
* 13,000 km³ (3,100 mile³ or 0.001%) is atmospheric water vapour at any given time. |
|||
Water is also found in the atmosphere in both liquid and vapor phases. The [[liquid water path]] is the measure of the amount of liquid water in an air column. |
|||
And so here is acting as an alkali, by receiving an H<sup>+</sup> ion. An acid donates a H+ ion, and water can also do this, such as the reaction with NaOH: |
|||
===Drinking water in politics=== |
|||
:NH<sub>3</sub> + H<sub>2</sub>O → NH<sub>4</sub><sup>+</sup> + OH<sup>-</sup> |
|||
[[Image:Drinkingwater.JPG|thumb|Drinking water]] |
|||
[[UNESCO]]'s World Water Development Report (WWDR, 2003) from its World Water Assessment Program indicates that in the next 20 years the world is facing an unprecedented lack of drinking water. The quantity of water available to everyone is predicted to decrease by 30%. The causes are contamination, [[global warming]] and political problems. |
|||
40% of the world's inhabitants have insufficient fresh water for minimal [[hygiene]]. More than 2.2 million people died in [[2000]] from [[disease]]s related to the consumption of contaminated water. In 2004, the [[United Kingdom|UK]] [[charity]] [[WaterAid]] reported that a child dies every 15 seconds due to easily preventable water-related diseases. |
|||
===pH in Practice=== |
|||
In theory, pure water has a pH of 7. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will rapidly dissolve carbon dioxide, forming a solution of carbonic acid, with a limiting pH of ~5.7 (reference: Kendall, J. (1916), ''Journal of the American Chemical Society'' 38 (11): 2460-2466). |
|||
The report indicates large global disparities in the raw volume of available water: from 10 m³ per person per year in [[Kuwait]] to 812.121 m³ in [[French Guiana]]. However, richer countries such as Kuwait can more easily cope with low water availability. |
|||
==Purifying water== |
|||
[[Image:Drinkingwater.JPG|thumb|Drinking water]] |
|||
In the [[United States]] [[water law]] is divided between two [[legal doctrine]]s: [[riparian water rights]], used in the eastern and southern states where there is an abundance of water, and the [[appropriation doctrine]] (or [[Colorado doctrine]]), used in the arid western states. |
|||
===Water in industry: purifying and wasting water=== |
|||
Purified water is needed for many industrial applications, as well as for consumption. Humans require water that does not contain too much salt or other impurities. Common impurities include chemicals or harmful [[bacteria]]. Some solutes are acceptable and even desirable for perceived taste enhancement. Water that is suitable for drinking is termed '''potable water'''. |
Purified water is needed for many industrial applications, as well as for consumption. Humans require water that does not contain too much salt or other impurities. Common impurities include chemicals or harmful [[bacteria]]. Some solutes are acceptable and even desirable for perceived taste enhancement. Water that is suitable for drinking is termed '''potable water'''. |
||
| Line 157: | Line 145: | ||
[[Image:Water.jpg|thumb|Shower]] |
[[Image:Water.jpg|thumb|Shower]] |
||
==Wasting water== |
|||
'''Wasting water''' is the misuse of water, i.e. using it unnecessarily. An example is the use of water, particularly water purified to human safe drinking standards, in unnecessary irrigation. Also, in homes, water may be wasted if the [[toilet]] is flushed unnecessarily or the tank leaks. Causing water to become [[pollution|polluted]] may be the biggest single misuse of water. To the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. However, in some cities, such as [[Hong Kong]], sea water is extensively used for flushing toilet citywide as a mean to conserve fresh water resources. |
'''Wasting water''' is the misuse of water, i.e. using it unnecessarily. An example is the use of water, particularly water purified to human safe drinking standards, in unnecessary irrigation. Also, in homes, water may be wasted if the [[toilet]] is flushed unnecessarily or the tank leaks. Causing water to become [[pollution|polluted]] may be the biggest single misuse of water. To the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. However, in some cities, such as [[Hong Kong]], sea water is extensively used for flushing toilet citywide as a mean to conserve fresh water resources. |
||
===Water as joke: Dihydrogen monoxide=== |
|||
==Mythology== |
|||
[[Chemist]]s sometimes jokingly refer to water as '''dihydrogen monoxide''' or '''[[DHMO]]''', the systematic covalent name of this molecule, especially in [[parody|parodies]] of chemical research that call for this "lethal chemical" to be banned. In 2004, the town of [[Aliso Viejo, California]] nearly banned foam cups after learning that DHMO was used in their production (see [http://slashdot.org/articles/04/03/16/1419252.shtml?tid=133&tid=186]). |
|||
Water is one of the four [[classical element]]s along with [[fire]], [[earth]] and [[air]], and was regarded as the [[ylem]], or basic stuff of the universe. Water was considered cold and moist. In the theory of the four [[bodily humour]]s, water was associated with [[phlegm]]. |
|||
The systematic acid name of water is '''hydroxic acid''' or '''hydroxilic acid''', although these terms are rarely used. |
|||
Water was also one of the [[Five Elements]] in [[China|Chinese]] [[Taoism]], along with [[earth]], [[fire]], [[wood]], and [[metal]]. |
|||
Likewise, the systematic alkali name of water is '''hydrogen hydroxide''' – both acid and alkali names exist for water because it is able to react both as an acid or an alkali, depending on the strength of the acid or alkali it is reacted with (it is [[amphoteric]]). |
|||
==Water in Religions== |
|||
===Water in religion=== |
|||
Water is considered a purifier in most religions, including [[Christianity]], [[Islam]], [[Judaism]], and [[Sikhism]]. For instance, [[baptism]] in Christian churches is done with water. As well, a ritual bath is done for the dead in pure water in many religions including Judaism and Islam. And in Islam, the daily [[Salah]] can only be done after [[Ablution]] ([[Wodoo]]) that is washing parts of the body in clean water. |
Water is considered a purifier in most religions, including [[Christianity]], [[Islam]], [[Judaism]], and [[Sikhism]]. For instance, [[baptism]] in Christian churches is done with water. As well, a ritual bath is done for the dead in pure water in many religions including Judaism and Islam. And in Islam, the daily [[Salah]] can only be done after [[Ablution]] ([[Wodoo]]) that is washing parts of the body in clean water. |
||
==Dihydrogen monoxide== |
|||
[[Chemist]]s sometimes jokingly refer to water as '''dihydrogen monoxide''' or '''[[DHMO]]''', the systematic covalent name of this molecule, especially in [[parody|parodies]] of chemical research that call for this "lethal chemical" to be banned. In 2004, the town of [[Aliso Viejo, California]] nearly banned foam cups after learning that DHMO was used in their production (see [http://slashdot.org/articles/04/03/16/1419252.shtml?tid=133&tid=186]). |
|||
==Physics and chemistry of water== |
|||
The systematic acid name of water is '''hydroxic acid''' or '''hydroxilic acid''', although these terms are rarely used. |
|||
===Dipolar nature of water=== |
|||
Likewise, the systematic alkali name of water is '''hydrogen hydroxide''' – both acid and alkali names exist for water because it is able to react both as an acid or an alkali, depending on the strength of the acid or alkali it is reacted with (it is [[amphoteric]]). |
|||
An important feature of water is its [[polar molecule|polar]] nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher [[electronegativity]] than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a [[dipole]]. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as [[hydrogen bond]]ing, and explains many of the properties of water. |
|||
This relatively weak (relative to the covalent bonds within the water molecule itself) attraction results in physical properties such as a relatively high [[boiling point]], because a lot of [[heat]] energy is necessary to break the hydrogen bonds between molecules. For example, [[sulfur]] is the element below oxygen in the periodic table, and its equivalent compound, hydrogen sulfide (H<sub>2</sub>S) does not have hydrogen bonds, and though it has twice the molecular weight of water, it is a gas at [[room temperature]]. The extra bonding between water molecules also gives liquid water a large [[specific heat capacity]]. |
|||
==Water rights and development== |
|||
[[UNESCO]]'s World Water Development Report (WWDR, 2003) from its World Water Assessment Program indicates that in the next 20 years the world is facing an unprecedented lack of drinking water. The quantity of water available to everyone is predicted to decrease by 30%. The causes are contamination, [[global warming]] and political problems. |
|||
Hydrogen bonding also gives water its unusual behaviour when freezing. When cooled to near freezing point, the presence of hydrogen bonds means that the molecules, as they rearrange to minimise their energy, form a structure that is actually of lower density: hence the solid form, ice, will float in water. In other words, water expands as it freezes (most other materials shrink on solidification). |
|||
40% of the world's inhabitants have insufficient fresh water for minimal [[hygiene]]. More than 2.2 million people died in [[2000]] from [[disease]]s related to the consumption of contaminated water. In 2004, the [[United Kingdom|UK]] [[charity]] [[WaterAid]] reported that a child dies every 15 seconds due to easily preventable water-related diseases. |
|||
Another consequence is that ice will melt if sufficient pressure is applied. |
|||
The report indicates large global disparities in the raw volume of available water: from 10 m³ per person per year in [[Kuwait]] to 812.121 m³ in [[French Guiana]]. However, richer countries such as Kuwait can more easily cope with low water availability. |
|||
===Water as a solvent=== |
|||
In the [[United States]] [[water law]] is divided between two [[legal doctrine]]s: [[riparian water rights]], used in the eastern and southern states where there is an abundance of water, and the [[appropriation doctrine]] (or [[Colorado doctrine]]), used in the arid western states. |
|||
Water is also a good [[solvent]] due to its polarity. When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small size of water molecules typically allows many water molecules to surround one molecule of ''solute''. The partially negative dipoles of the water are attracted to positively charged components of the solute, and vice versa for the positive dipoles. |
|||
==Cloth and clothing== |
|||
In general, ionic and polar substances such as [[acid]]s, [[alcohol]]s, and [[salt]]s are easily soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in [[van der Waals force|van der Waals interactions]] with nonpolar molecules. |
|||
Some [[cloth]]s such as a [[towel]] are specially made for absorbing water. |
|||
An example of an ionic solute is [[sodium chloride|table salt]]; the sodium chloride, NaCl, separates into Na<sup>+</sup> [[cation]]s and Cl<sup>-</sup> [[anion]]s, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is [[sucrose|table sugar]]. The water dipoles hydrogen bond to the dipolar regions of the sugar molecule and allow it to be carried away into solution. |
|||
Some [[clothing]] is for protection against water (e.g. [[raincoat]]), or to be used in water ([[swimsuit]]). See also [[Clothing#Inappropriateness_and_clothing|inappropriateness and clothing]] and [[wet T-shirt contest]]. |
|||
===Amphoteric nature of water=== |
|||
Chemically, water is [[amphoteric]]: able to act as an acid or base. Occasionally the term ''hydroxic acid'' is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of [[hydroxide]] ions (OH<sup>-</sup>) is equal to that of the [[hydronium]] (H<sub>3</sub>O<sup>+</sup>) or [[hydrogen]] ions (H<sup>+</sup>) ions. If the [[equilibrium]] is disturbed, the solution becomes acidic (higher concentration of hydronium ions) or basic (higher concentration of hydroxide ions). |
|||
Water can act as either an acid or an alkali in reactions. According to the [[Brønsted-Lowry]] system, an acid defined as a species which donates a proton (a H<sup>+</sup> ion) in a reaction, and an alkali as something which receives a proton. When reacting with a stronger acid, water acts as an alkali, and will act as an acid when reaction with a weaker acid. For instance, it will receive a H+ from HCl in the equilibrium: |
|||
:HCl + H<sub>2</sub>O → H<sub>3<sub>O<sup>+</sup> + Cl<sup>-</sup> |
|||
And so here is acting as an alkali, by receiving an H<sup>+</sup> ion. An acid donates a H+ ion, and water can also do this, such as the reaction with NaOH: |
|||
:NH<sub>3</sub> + H<sub>2</sub>O → NH<sub>4</sub><sup>+</sup> + OH<sup>-</sup> |
|||
==History== |
|||
==Mythology== |
|||
Water is one of the four [[classical element]]s along with [[fire]], [[earth]] and [[air]], and was regarded as the [[ylem]], or basic stuff of the universe. Water was considered cold and moist. In the theory of the four [[bodily humour]]s, water was associated with [[phlegm]]. |
|||
Water was also one of the [[Five Elements]] in [[China|Chinese]] [[Taoism]], along with [[earth]], [[fire]], [[wood]], and [[metal]]. |
|||
== See also == |
== See also == |
||
| Line 227: | Line 230: | ||
* [http://www.rsd-solar.com/ Water distillation using only the sun] |
* [http://www.rsd-solar.com/ Water distillation using only the sun] |
||
* [http://www.siwi.org/ Stockholm International Water Institute] (SIWI) |
* [http://www.siwi.org/ Stockholm International Water Institute] (SIWI) |
||
[[ar:ماء]] |
[[ar:ماء]] |
||
[[ca:Aigua]] |
[[ca:Aigua]] |
||
Revision as of 19:34, 15 September 2004
| General | |
|---|---|
| Name | Water |
| Diagram | 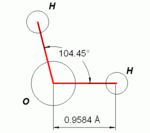
|
| Chemical formula | H2O |
| Appearance | Colourless liquid |
| Physical | |
| Formula weight | 18.01528 amu |
| Melting point | 273.15 K (0 °C) |
| Boiling point | 373.15 K (100 °C) |
| Critical temperature | 674 K |
| Critical pressure | 2.21 × 107 Pa |
| Density | 1.0 ×103 kg/m3 at 4°C |
| Thermochemistry | |
| ΔfH0gas | -241.83 kJ/mol |
| ΔfH0liquid | -285.83 kJ/mol |
| ΔfH0solid | -291.83 kJ/mol |
| S0gas, 1 bar | 188.84 J/mol·K |
| S0liquid, 1 bar | 69.95 J/mol·K |
| S0solid | 41 J/mol·K |
| Safety | |
| Ingestion | Necessary to life; excessive consumption can cause headache, confusion, and cramps, and can be fatal in athletes |
| Inhalation | Non-toxic. Can dissolve surfactant of lungs. Suffocation in water is called drowning. |
| Skin | Prolonged immersion may cause flaking (desquamation). |
| Eyes | Not dangerous. |
|
SI units were used where possible. Unless otherwise stated, standard conditions were used. | |
Water is a substance found almost everywhere on earth and is required by all known life. About 70% of the Earth's surface is covered by water. Water is known to exist, in ice form, on several other bodies in the solar system and beyond. The importance of water to terrestrial life has led many to surmise that its existence in liquid form anywhere besides Earth could provide a viable habitat for extraterrestrial life.
Water is liquid at standard temperature and pressure. Its other common forms are ice, snow and vapor. It has the chemical formula H2O, meaning that one molecule of water is composed of two hydrogen atoms and one oxygen atom.
Water in practice
Forms of water
- See the category: Category:Forms of water
The solid state of water is known as ice; the gaseous state is known as water vapor (or steam).
Water exhibits some very strange behaviors, including the formation of states such as vitreous ice, a noncrystalline (glassy), solid state of water.
At temperatures greater than 647 K and pressures greater than 22.064 MPa, a collection of water molecules assumes a supercritical condition, in which liquid-like clusters float within a vapor-like phase.
Water properties
Just like most other materials, liquid water becomes denser with lowering temperature. However, unlike most other materials, when cooled to near freezing point, it becomes lighter with lowering temperature. Liquid water reaches its highest density at a temperature of 4 °C.
This has an interesting consequence for water life in winter. Water chilled at the surface becomes denser and sinks, forming convection currents that cool the whole water body, but when the temperature of the lake water reaches 4°C, water on the surface, as it chills further, becomes less dense, and stays as a surface layer which eventually forms ice. Since downward convection of colder water is blocked by the density change, any large body of water frozen in winter will have the bulk of its water still liquid at 4°C beneath the icy surface, allowing fish to survive. This is one of the principal examples of finely-tuned physical properties that support life on Earth that is used as an argument for the anthropic principle.
The temperature and pressure at which solid, liquid, and gaseous water coexist in equilibrium is called the triple point of water. This point is used to define the units of temperature (formerly the degree Celsius and now the Kelvin). The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.2 Pa.
Water is also a good solvent. The solvent properties of water are vital in biology, because many biochemical reactions take place only within aqueous solutions (e.g., reactions in the cytoplasm and blood). In addition, water is used to transport biological molecules.
Water drops are stable thanks to the high surface tension of water. This can be seen when small quantities of water are put onto a nonsoluble surface: the water stays together as drops. This property is important for life, e.g. when water is carried through xylem up stems in plants : the strong intermolecular attractions hold the water column together, and prevent tension caused by transpiration pull. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem vessel inoperative.
Pure water is actually a good insulator (poor conductor), meaning that it does not conduct electricity well. Because water is such a good solvent, however, it often has some solute dissolved in it, most frequently salt. If water has such impurities, then it can conduct electricity much better, because impurities such as salt comprise free ions in aqueous solution by which an electric current can flow.
Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called electrolysis. Water molecules naturally disassociate into H+ and OH- ions, which are pulled toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H2 gas. At the anode, four OH- ions combine and release O2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected.
In theory, pure water has a pH of 7. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will rapidly dissolve carbon dioxide, forming a solution of carbonic acid, with a limiting pH of ~5.7.
Water on Earth
Liquid water is found in body of water, such as an ocean, sea, lake, river, stream, canal, pond, or the like. See water resources for information about fresh water supplies. Also see: sea water, fresh water, and underwater.
Earth's approximate water volume (the total water supply of the world) is 1,360,000,000 km³ (326,000,000 mile³). Of this volume:
- 1,320,000,000 km³ (316,900,000 mile³ or 97.2%) is in the oceans
- 25,000,000 km³ (6,000,000 mile³ or 1.8%) is in glaciers and icecaps
- 13,000,000 km³ (3,000,000 mile³ or 0.9%) is groundwater.
- 250,000 km³ (60,000 mile³ or 0.02%) is fresh water in lakes, inland seas, and rivers.
- 13,000 km³ (3,100 mile³ or 0.001%) is atmospheric water vapour at any given time.
Water is also found in the atmosphere in both liquid and vapor phases. The liquid water path is the measure of the amount of liquid water in an air column.
Drinking water in politics
UNESCO's World Water Development Report (WWDR, 2003) from its World Water Assessment Program indicates that in the next 20 years the world is facing an unprecedented lack of drinking water. The quantity of water available to everyone is predicted to decrease by 30%. The causes are contamination, global warming and political problems.
40% of the world's inhabitants have insufficient fresh water for minimal hygiene. More than 2.2 million people died in 2000 from diseases related to the consumption of contaminated water. In 2004, the UK charity WaterAid reported that a child dies every 15 seconds due to easily preventable water-related diseases.
The report indicates large global disparities in the raw volume of available water: from 10 m³ per person per year in Kuwait to 812.121 m³ in French Guiana. However, richer countries such as Kuwait can more easily cope with low water availability.
In the United States water law is divided between two legal doctrines: riparian water rights, used in the eastern and southern states where there is an abundance of water, and the appropriation doctrine (or Colorado doctrine), used in the arid western states.
Water in industry: purifying and wasting water
Purified water is needed for many industrial applications, as well as for consumption. Humans require water that does not contain too much salt or other impurities. Common impurities include chemicals or harmful bacteria. Some solutes are acceptable and even desirable for perceived taste enhancement. Water that is suitable for drinking is termed potable water.
Six popular methods for purifying water are:
- Filtering: Water is passed through a sieve that catches small particles. The tighter the mesh of the sieve, the smaller the particles must be to pass through. Filtering is not sufficient to completely purify water, but it is often a necessary first step, since such particles can interfere with the more thorough purification methods.
- Boiling: Water is heated to its boiling point long enough to inactivate or kill microorganisms that normally live in water at room temperature. In areas where the water is "hard", (containing dissolved calcium salts), boiling decomposes the bicarbonate ion, resulting in some (but not all) of the dissolved calcium being precipitated in the form of calcium carbonate. This is the so-called "fur" that builds up on kettle elements etc. in hard water areas. With the exception of calcium, boiling does not remove solutes of higher boiling point than water, and in fact increases their concentration (due to some water being lost as vapour)
- Carbon filtering: Charcoal, a form of carbon with a high surface area due to its mode of preparation, adsorbs many compounds, including some toxic compounds. Water is passed through activated charcoal to remove such contaminants. This method is most commonly used in household water filters and fish tanks. Household filters for drinking water sometimes also contain silver, trace amounts of silver ions having a bactericidal effect.
- Distilling: Distillation involves boiling the water to produce water vapour. The water vapour then rises to a cooled surface where it can condense back into a liquid and be collected. Because the solutes are not normally vaporized, they remain in the boiling solution. Even distillation does not completely purify water, because of contaminants with similar boiling points and droplets of unvaporized liquid carried with the steam. However, 99.9% pure water can be obtained by distillation.
- Reverse osmosis: Mechanical pressure is applied to an impure solution to force pure water through a semi-permeable membrane. The term is reverse osmosis, because normal osmosis would result in pure water moving in the other direction to dilute the impurities. Reverse osmosis is theoretically the most thorough method of large-scale water purification available, although perfect semi-permable membranes are difficult to create.
- Ion exchange: Most common ion exchange systems use a zeolite resin bed and simply remove unwanted ions (Ca2+ and Mg2+ ) with benign (soap friendly) sodium ions. This is the common water softener. A more rigorous type of ion exchange swaps hydrogen, H+, ions for unwanted cations and hydroxide, OH-, for unwanted anions. The result is H+ + OH- ==> H2O. This system is recharged with hydrochloric acid and sodium hydroxide. The result is essentially deionized water.
- Electrodeionization: Water is passed between a positive electrode and a negative electrode. Ion selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity de-ionized water results. The water is usually passed through a reverse osmosis unit first to remove nonionic organic contaminants.

Wasting water is the misuse of water, i.e. using it unnecessarily. An example is the use of water, particularly water purified to human safe drinking standards, in unnecessary irrigation. Also, in homes, water may be wasted if the toilet is flushed unnecessarily or the tank leaks. Causing water to become polluted may be the biggest single misuse of water. To the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. However, in some cities, such as Hong Kong, sea water is extensively used for flushing toilet citywide as a mean to conserve fresh water resources.
Water as joke: Dihydrogen monoxide
Chemists sometimes jokingly refer to water as dihydrogen monoxide or DHMO, the systematic covalent name of this molecule, especially in parodies of chemical research that call for this "lethal chemical" to be banned. In 2004, the town of Aliso Viejo, California nearly banned foam cups after learning that DHMO was used in their production (see [1]).
The systematic acid name of water is hydroxic acid or hydroxilic acid, although these terms are rarely used.
Likewise, the systematic alkali name of water is hydrogen hydroxide – both acid and alkali names exist for water because it is able to react both as an acid or an alkali, depending on the strength of the acid or alkali it is reacted with (it is amphoteric).
Water in religion
Water is considered a purifier in most religions, including Christianity, Islam, Judaism, and Sikhism. For instance, baptism in Christian churches is done with water. As well, a ritual bath is done for the dead in pure water in many religions including Judaism and Islam. And in Islam, the daily Salah can only be done after Ablution (Wodoo) that is washing parts of the body in clean water.
Physics and chemistry of water
Dipolar nature of water
An important feature of water is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a dipole. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as hydrogen bonding, and explains many of the properties of water.
This relatively weak (relative to the covalent bonds within the water molecule itself) attraction results in physical properties such as a relatively high boiling point, because a lot of heat energy is necessary to break the hydrogen bonds between molecules. For example, sulfur is the element below oxygen in the periodic table, and its equivalent compound, hydrogen sulfide (H2S) does not have hydrogen bonds, and though it has twice the molecular weight of water, it is a gas at room temperature. The extra bonding between water molecules also gives liquid water a large specific heat capacity.
Hydrogen bonding also gives water its unusual behaviour when freezing. When cooled to near freezing point, the presence of hydrogen bonds means that the molecules, as they rearrange to minimise their energy, form a structure that is actually of lower density: hence the solid form, ice, will float in water. In other words, water expands as it freezes (most other materials shrink on solidification).
Another consequence is that ice will melt if sufficient pressure is applied.
Water as a solvent
Water is also a good solvent due to its polarity. When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute. The partially negative dipoles of the water are attracted to positively charged components of the solute, and vice versa for the positive dipoles.
In general, ionic and polar substances such as acids, alcohols, and salts are easily soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with nonpolar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl- anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles hydrogen bond to the dipolar regions of the sugar molecule and allow it to be carried away into solution.
Amphoteric nature of water
Chemically, water is amphoteric: able to act as an acid or base. Occasionally the term hydroxic acid is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of hydroxide ions (OH-) is equal to that of the hydronium (H3O+) or hydrogen ions (H+) ions. If the equilibrium is disturbed, the solution becomes acidic (higher concentration of hydronium ions) or basic (higher concentration of hydroxide ions).
Water can act as either an acid or an alkali in reactions. According to the Brønsted-Lowry system, an acid defined as a species which donates a proton (a H+ ion) in a reaction, and an alkali as something which receives a proton. When reacting with a stronger acid, water acts as an alkali, and will act as an acid when reaction with a weaker acid. For instance, it will receive a H+ from HCl in the equilibrium:
- HCl + H2O → H3O+ + Cl-
And so here is acting as an alkali, by receiving an H+ ion. An acid donates a H+ ion, and water can also do this, such as the reaction with NaOH:
- NH3 + H2O → NH4+ + OH-
History
Mythology
Water is one of the four classical elements along with fire, earth and air, and was regarded as the ylem, or basic stuff of the universe. Water was considered cold and moist. In the theory of the four bodily humours, water was associated with phlegm.
Water was also one of the Five Elements in Chinese Taoism, along with earth, fire, wood, and metal.
See also
- Mineral water
- Dehydration
- Dihydrogen monoxide (DHMO)
- Double distilled water
- Drought
- Evapotranspiration
- Fresh water
- Flood
- Heavy water
- Holy water
- Hydrography
- Hydrology
- Irrigation
- Mpemba effect - can hot water freeze faster than cold water?
- Polywater theory
- Precipitation
- Rain
- Sea water
- Trasvasement
- Wastewater
- Water quality
- Water resources
External links
- World Water Forum
- World Water Assessment Program
- United Nations' World Water Development Report
- United Nations GEMS/Water Programme
- Water Structure and Behaviour
- SAHRA - Global Water Newswatch
- A spoof site on the "dangers" of dihydrogen monoxide
- Water distillation using only the sun
- Stockholm International Water Institute (SIWI)
