Cucurbitacin: Difference between revisions
m →See also: refpunct. |
repeat ref; copyedits; change subhead and add research/toxicity discussion by Citation bot |
||
| Line 1: | Line 1: | ||
[[File:Cucurbitane Grundstruktur num.svg|right|thumb|200px|Cucurbita-5-ene with standard carbon numbering.]] |
[[File:Cucurbitane Grundstruktur num.svg|right|thumb|200px|Cucurbita-5-ene with standard carbon numbering.]] |
||
'''Cucurbitacin''' is any of a class of [[biochemistry|biochemical]] compounds that some |
'''Cucurbitacin''' is any of a class of [[biochemistry|biochemical]] compounds that some plants — notably members of the family [[Cucurbitaceae]], which includes the common [[pumpkin]]s and [[gourd]]s — produce and which function as a defence against [[herbivore]]s. Cucurbitacins are chemically classified as [[steroid]]s, formally derived from [[cucurbitane]], a [[triterpene]] [[hydrocarbon]]—specifically, from the unsaturated variant [[cucurbita-5-ene]], or 19-(10→9β)-''abeo''-10α-lanost-5-ene. They often occur as [[glycoside]]s.<ref name=ChenRev>Jian Chao Chen, Ming Hua Chiu, Rui Lin Nie, Geoffrey A. Cordell and Samuel X. Qiu (2005), "Cucurbitacins and cucurbitane glycosides: structures and biological activities" ''Natural Product Reports, volume 22, pages 386-399 {{doi|10.1039/B418841C}}</ref> They and their derivatives have been found in many plant families (including [[Brassicaceae]], [[Cucurbitaceae]], [[Scrophulariaceae]], [[Begoniaceae]], [[Elaeocarpaceae]], [[Datiscaceae]], [[Desfontainiaceae]], [[Polemoniaceae]], [[Primulaceae]], [[Rubiaceae]], [[Sterculiaceae]], [[Rosaceae]], and [[Thymelaeaceae]]), in some [[mushroom]]s (including Russula and Hebeloma) and even in some marine mollusks. |
||
Cucurbitacins may be a taste deterrent in plants foraged by some animals and in some edible plants preferred by humans, like [[cucumber]]s. In [[basic research|laboratory research]], cucurbitacins have [[cytotoxic]] properties and are under study for their potential biological activities.<ref>{{cite journal|pmid=23559908|year=2013|last1=Alghasham|first1=AA|title=Cucurbitacins - a promising target for cancer therapy|volume=7|issue=1|pages=77–89|pmc=3612419|journal=International Journal of Health Sciences|doi=10.12816/0006025}}</ref><ref>{{cite journal|pmid=23350897|year=2013|last1=Kapoor|first1=S|title=Cucurbitacin B and Its Rapidly Emerging Role in the Management of Systemic Malignancies Besides Lung Carcinomas|doi=10.1089/cbr.2012.1373|journal=Cancer Biotherapy |
Cucurbitacins may be a taste deterrent in plants foraged by some animals and in some edible plants preferred by humans, like [[cucumber]]s. In [[basic research|laboratory research]], cucurbitacins have [[cytotoxic]] properties and are under study for their potential biological activities.<ref name="ijhs">{{cite journal|pmid=23559908|year=2013|last1=Alghasham|first1=AA|title=Cucurbitacins - a promising target for cancer therapy|volume=7|issue=1|pages=77–89|pmc=3612419|journal=International Journal of Health Sciences|doi=10.12816/0006025}}</ref><ref name="kapoor">{{cite journal|pmid=23350897|year=2013|last1=Kapoor|first1=S|title=Cucurbitacin B and Its Rapidly Emerging Role in the Management of Systemic Malignancies Besides Lung Carcinomas|doi=10.1089/cbr.2012.1373|journal=Cancer Biotherapy and Radiopharmaceuticals|volume=28|issue=4|pages=359}}</ref> |
||
== Biosynthesis == |
== Biosynthesis == |
||
The biosynthesis of |
The biosynthesis of cucurbitacin C has been described. Zhang et al. (2014) identified nine [[cucumber]] genes in the pathway for biosynthesis of cucurbitacin C and elucidated four catalytic steps.<ref name="Zhang2014">{{cite journal | last1 = Zhang | first1 = Y. | display-authors = etal | year = 2014 | title = Biosynthesis, regulation, and domestication of bitterness in cucumber | url = http://www.sciencemag.org/content/346/6213/1084.full.html | journal = Science | volume = 346 | issue = 6213| pages = 1084–1088 | doi=10.1126/science.1259215 | pmid=25430763| bibcode = 2014Sci...346.1084S }}</ref> These authors also discovered the [[Transcription factors, general|transcription factors]] ''Bl'' (Bitter leaf) and ''Bt'' (Bitter fruit) that regulate this pathway in leaves and fruits, respectively. The Bi gene confers bitterness to the entire plant and is genetically associated with an [[operon]]-like gene cluster, similar to the gene cluster involved in [[Thalianol synthase|thalianol biosynthesis]] in ''[[Arabidopsis]]''. Fruit bitterness requires both Bi and the dominant Bt (Bitter fruit) gene. Nonbitterness of cultivated cucumber fruit is conferred by bt, an allele selected during domestication. Bi is a member of the oxidosqualene cyclase (OSC) gene family. Phylogenetic analysis showed that Bi is the [[Homology (biology)|ortholog]] of cucurbitadienol synthase gene CPQ in squash (''[[Cucurbita pepo]]'') <ref name="Zhang2014"/> |
||
== Variants == |
== Variants == |
||
| Line 132: | Line 132: | ||
Several other cucurbitacins have been found in plants.<ref name=ChenRev/>{{rp|152–156''','''164–165}}<!--SO let's list them all--> |
Several other cucurbitacins have been found in plants.<ref name=ChenRev/>{{rp|152–156''','''164–165}}<!--SO let's list them all--> |
||
==Occurrence== |
==Occurrence and bitter taste== |
||
Constituents of the [[colocynth]] fruit and leaves (''[[Citrullus colocynthis]]'') include cucurbitacins.<ref>{{cite journal|pmid=25761128|year=2015|author1=Song|first1=F|title=Two new cucurbitane-type triterpenoid saponins isolated from ethyl acetate extract of Citrullus colocynthis fruit|journal=Journal of Asian Natural Products Research|volume=17|issue=8|pages=813–8|last2=Dai|first2=B|last3=Zhang|first3=H. Y.|last4=Xie|first4=J. W.|last5=Gu|first5=C. Z.|last6=Zhang|first6=J|doi=10.1080/10286020.2015.1015999}}</ref><ref>{{cite journal|pmid=26437392|year=2015|author1=Chawech|first1=R|title=Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad|journal=Molecules|volume=20|issue=10|pages=18001–15|last2=Jarraya|first2=R|last3=Girardi|first3=C|last4=Vansteelandt|first4=M|last5=Marti|first5=G|last6=Nasri|first6=I|last7=Racaud-Sultan|first7=C|last8=Fabre|first8=N|doi=10.3390/molecules201018001}}</ref> |
Constituents of the [[colocynth]] fruit and leaves (''[[Citrullus colocynthis]]'') include cucurbitacins.<ref>{{cite journal|pmid=25761128|year=2015|author1=Song|first1=F|title=Two new cucurbitane-type triterpenoid saponins isolated from ethyl acetate extract of Citrullus colocynthis fruit|journal=Journal of Asian Natural Products Research|volume=17|issue=8|pages=813–8|last2=Dai|first2=B|last3=Zhang|first3=H. Y.|last4=Xie|first4=J. W.|last5=Gu|first5=C. Z.|last6=Zhang|first6=J|doi=10.1080/10286020.2015.1015999}}</ref><ref>{{cite journal|pmid=26437392|year=2015|author1=Chawech|first1=R|title=Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad|journal=Molecules|volume=20|issue=10|pages=18001–15|last2=Jarraya|first2=R|last3=Girardi|first3=C|last4=Vansteelandt|first4=M|last5=Marti|first5=G|last6=Nasri|first6=I|last7=Racaud-Sultan|first7=C|last8=Fabre|first8=N|doi=10.3390/molecules201018001}}</ref> The 2-O-β-D-[[glucopyranoside]]s of cucurbitacins K and L can be extracted with [[ethanol]] from fruits of ''[[Cucurbita pepo]]'' [[cultivar|cv]] ''dayangua''.<ref name=DCWang2007/> Pentanorcucurbitacins A and B can be extracted with [[methanol]] from the stems of ''[[Momordica charantia]]''.<ref name=CRChen2010>{{cite journal | last1 = Chen | first1 = Chiy-Rong | last2 = Liao | first2 = Yun-Wen | last3 = Wang | first3 = Lai | last4 = Kuo | first4 = Yueh-Hsiung | last5 = Liu | first5 = Hung-Jen | last6 = Shih | first6 = Wen-Ling | last7 = Cheng | first7 = Hsueh-Ling | last8 = Chi-I | first8 = Chang | year = 2010 | title = Cucurbitane Triterpenoids from ''Momordica charantia'' and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells | url = | journal = Chemical & Pharmaceutical Bulletin | volume = 58 | issue = 12| pages = 1639–1642 | doi = 10.1248/cpb.58.1639 }}</ref> Cucurbitacins B and I, and derivatives of cucurbitacins B, D and E, can be extracted with methanol from dried tubers of ''Hemsleya endecaphylla''.<ref name=JCChen2008oc>{{cite journal | last1 = Chen | first1 = Jian-Chao | last2 = Zhang | first2 = Gao-Hong | last3 = Zhang | first3 = Zhong-Quan | last4 = Qiu | first4 = Ming-Hua | last5 = Zheng | first5 = Yong-Tang | last6 = Yang | first6 = Liu-Meng | last7 = Yu | first7 = Kai-Bei | year = 2008 | title = Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers of ''Hemsleya endecaphylla'' with HIV-1 Inhibitory Activity | url = | journal = J. Nat. Prod. | volume = 71 | issue = 1| pages = 153–155 | doi = 10.1021/np0704396 | pmid=18088099}}</ref> |
||
| ⚫ | Cucurbitacins impart a bitter taste in plant foods such as [[cucumber]], [[zucchini]], [[melon]] and [[pumpkin]].<ref name=kaushik/><ref>{{cite journal|pmid=25430763|year=2014|url=http://science.sciencemag.org/content/346/6213/1084.long|author1=Shang|first1=Y|title=Plant science. Biosynthesis, regulation, and domestication of bitterness in cucumber|journal=Science|volume=346|issue=6213|pages=1084–8|last2=Ma|first2=Y|last3=Zhou|first3=Y|last4=Zhang|first4=H|last5=Duan|first5=L|last6=Chen|first6=H|last7=Zeng|first7=J|last8=Zhou|first8=Q|last9=Wang|first9=S|last10=Gu|first10=W|last11=Liu|first11=M|last12=Ren|first12=J|last13=Gu|first13=X|last14=Zhang|first14=S|last15=Wang|first15=Y|last16=Yasukawa|first16=K|last17=Bouwmeester|first17=H. J.|last18=Qi|first18=X|last19=Zhang|first19=Z|last20=Lucas|first20=W. J.|last21=Huang|first21=S|doi=10.1126/science.1259215|bibcode=2014Sci...346.1084S}}</ref> |
||
The 2-O-β-D-[[glucopyranoside]]s of cucurbitacins K and L can be extracted with [[ethanol]] from fruits of ''[[Cucurbita pepo]]'' [[cultivar|cv]] ''dayangua''.<ref name=DCWang2007/> |
|||
==Research and toxicity== |
|||
Pentanorcucurbitacins A and B can be extracted with [[methanol]] from the stems of ''[[Momordica charantia]]''.<ref name=CRChen2010>{{cite journal | last1 = Chen | first1 = Chiy-Rong | last2 = Liao | first2 = Yun-Wen | last3 = Wang | first3 = Lai | last4 = Kuo | first4 = Yueh-Hsiung | last5 = Liu | first5 = Hung-Jen | last6 = Shih | first6 = Wen-Ling | last7 = Cheng | first7 = Hsueh-Ling | last8 = Chi-I | first8 = Chang | year = 2010 | title = Cucurbitane Triterpenoids from ''Momordica charantia'' and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells | url = | journal = Chemical & Pharmaceutical Bulletin | volume = 58 | issue = 12| pages = 1639–1642 | doi = 10.1248/cpb.58.1639 }}</ref> |
|||
Cucurbitacins are under [[basic research]] for their biological properties, including [[toxicity]] and potential [[pharmacology|pharmacological]] uses in development of drugs for [[inflammation]], [[cancer]], [[cardiovascular diseases]], and [[diabetes]], among others.<ref name=ChenRev/><ref name=ijhs/><ref name=kapoor/><ref name="kaushik">{{cite journal|pmc=4441156|year=2015|author1=Kaushik|first1=U|title=Cucurbitacins – an insight into medicinal leads from nature|journal=Pharmacognosy Reviews|volume=9|issue=17|pages=12–18|last2=Aeri|first2=V|last3=Mir|first3=S. R|doi=10.4103/0973-7847.156314}}</ref> |
|||
| ⚫ | Pathologists found cucurbitacin in the stomach of a 79-year-old man who died in [[Bavaria]], [[Germany]], shortly after eating a casserole containing [[zucchini]] he had received from a neighbor.<ref>{{cite web|title=Mann stirbt an Garten Zucchini|url=http://www.sueddeutsche.de/panorama/vergiftung-mann-stirbt-an-garten-zucchini-1.2615508|accessdate=24 August 2015}}</ref><ref>{{cite web|title=Auf den Geschmack kommt es an|url=http://www.sueddeutsche.de/panorama/giftige-bitterstoffe-in-gemuese-auf-den-geschmack-kommt-es-an-1.2616420|accessdate=24 August 2015}}</ref> |
||
Cucurbitacins B and I, and derivatives of cucurbitacins B, D and E, can be extracted with methanol from dried tubers of ''Hemsleya endecaphylla''.<ref name=JCChen2008oc>{{cite journal | last1 = Chen | first1 = Jian-Chao | last2 = Zhang | first2 = Gao-Hong | last3 = Zhang | first3 = Zhong-Quan | last4 = Qiu | first4 = Ming-Hua | last5 = Zheng | first5 = Yong-Tang | last6 = Yang | first6 = Liu-Meng | last7 = Yu | first7 = Kai-Bei | year = 2008 | title = Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers of ''Hemsleya endecaphylla'' with HIV-1 Inhibitory Activity | url = | journal = J. Nat. Prod. | volume = 71 | issue = 1| pages = 153–155 | doi = 10.1021/np0704396 | pmid=18088099}}</ref> |
|||
==Bitter taste== |
|||
| ⚫ | Cucurbitacins impart a bitter taste in plant foods such as cucumber, melon and pumpkin.<ref>{{cite journal|pmid=25430763|year=2014|url=http://science.sciencemag.org/content/346/6213/1084.long|author1=Shang|first1=Y|title=Plant science. Biosynthesis, regulation, and domestication of bitterness in cucumber|journal=Science|volume=346|issue=6213|pages=1084–8|last2=Ma|first2=Y|last3=Zhou|first3=Y|last4=Zhang|first4=H|last5=Duan|first5=L|last6=Chen|first6=H|last7=Zeng|first7=J|last8=Zhou|first8=Q|last9=Wang|first9=S|last10=Gu|first10=W|last11=Liu|first11=M|last12=Ren|first12=J|last13=Gu|first13=X|last14=Zhang|first14=S|last15=Wang|first15=Y|last16=Yasukawa|first16=K|last17=Bouwmeester|first17=H. J.|last18=Qi|first18=X|last19=Zhang|first19=Z|last20=Lucas|first20=W. J.|last21=Huang|first21=S|doi=10.1126/science.1259215}}</ref> |
||
==Deaths== |
|||
| ⚫ | Pathologists found cucurbitacin in the stomach of a 79-year-old man who died in [[Bavaria]], [[Germany]], shortly after eating a casserole containing [[zucchini]] he had received from a neighbor |
||
== See also == |
== See also == |
||
Revision as of 23:21, 17 October 2017
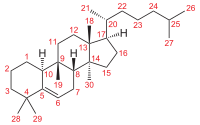
Cucurbitacin is any of a class of biochemical compounds that some plants — notably members of the family Cucurbitaceae, which includes the common pumpkins and gourds — produce and which function as a defence against herbivores. Cucurbitacins are chemically classified as steroids, formally derived from cucurbitane, a triterpene hydrocarbon—specifically, from the unsaturated variant cucurbita-5-ene, or 19-(10→9β)-abeo-10α-lanost-5-ene. They often occur as glycosides.[1] They and their derivatives have been found in many plant families (including Brassicaceae, Cucurbitaceae, Scrophulariaceae, Begoniaceae, Elaeocarpaceae, Datiscaceae, Desfontainiaceae, Polemoniaceae, Primulaceae, Rubiaceae, Sterculiaceae, Rosaceae, and Thymelaeaceae), in some mushrooms (including Russula and Hebeloma) and even in some marine mollusks.
Cucurbitacins may be a taste deterrent in plants foraged by some animals and in some edible plants preferred by humans, like cucumbers. In laboratory research, cucurbitacins have cytotoxic properties and are under study for their potential biological activities.[2][3]
Biosynthesis
The biosynthesis of cucurbitacin C has been described. Zhang et al. (2014) identified nine cucumber genes in the pathway for biosynthesis of cucurbitacin C and elucidated four catalytic steps.[4] These authors also discovered the transcription factors Bl (Bitter leaf) and Bt (Bitter fruit) that regulate this pathway in leaves and fruits, respectively. The Bi gene confers bitterness to the entire plant and is genetically associated with an operon-like gene cluster, similar to the gene cluster involved in thalianol biosynthesis in Arabidopsis. Fruit bitterness requires both Bi and the dominant Bt (Bitter fruit) gene. Nonbitterness of cultivated cucumber fruit is conferred by bt, an allele selected during domestication. Bi is a member of the oxidosqualene cyclase (OSC) gene family. Phylogenetic analysis showed that Bi is the ortholog of cucurbitadienol synthase gene CPQ in squash (Cucurbita pepo) [4]
Variants
The cucurbitacins include:
Cucurbitacin A
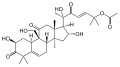
- Cucurbitacin A found in some species of Cucumis [1]: 1
- Pentanorcucurbitacin A, or 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one C
25H
40O
2, white powder[5]: 1
- Pentanorcucurbitacin A, or 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one C
Cucurbitacin B
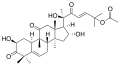
- Cucurbitacin B from Hemsleya endecaphylla (62 mg/72 g)[6]: 4 and other plants (e.g. Cucurbita andreana[7]); anti-inflammatory, any-hepatotoxic [1]: 2
- Cucurbitacin B 2-O-glucoside, from Begonia heracleifolia [1]: 3
- 23,24-Dihydrocucurbitacin B from Hemsleya endecaphylla, 49 mg/72 g[6]: 5
- 23,24-Dihydrocucurbitacin B 2-O-glucoside from roots of Picrorhiza kurrooa [1]: 4
- Deacetoxycucurbitacin B 2-O-glucoside from roots of Picrorhiza kurrooa [1]: 5
- Isocucurbitacin B, from Echinocystis fabacea [1]: 6
- 23,24-Dihydroisocucurbitacin B 3-glucoside from Wilbrandia ebracteata [1]: 7
- 23,24-Dihydro-3-epi-isocucurbitacin B, from Bryonia verrucosa [1]: 8
- Pentanorcucurbitacin B or 3,7-dioxo-23,24,25,26,27-pentanorcucurbit-5-en-22-oic acid, C
25H
36O
4, white powder[5]: 2
Cucurbitacin C
- Cucurbitacin C, from Cucumis sativus (Cucumber) [1]: 11
Cucurbitacin D

- Cucurbitacin D, from Trichosanthes kirilowii and many other plants [1] (e.g. Cucurbita andreana[7]): 12
- 3-Epi-isocucurbitacin D, from species of Physocarpus [1]: 14 and Phormium tenax[8]
- 22-Deoxocucurbitacin D from Hemsleya endecaphylla, 14 mg/72 g[6]: 6
- 23,24-Dihydrocucurbitacin D from Trichosanthes kirilowii [1]: 13 also from H. endecaphylla, 80 mg/72 g[6]: 3
- 23,24-Dihydro-epi-isocucurbitacin D, from Acanthosicyos horridus [1]: 20
- 22-Deoxocucurbitacin D from Wilbrandia ebracteata [1]: 21
- Anhydro-22-deoxo-3-epi-isocucurbitacin D from Ecballium elaterium [1]: 22
- 25-O-Acetyl-2-deoxycucurbitacin D (amarinin) from Luffa amara [1]: 24
- 2-Deoxycucurbitacin D, from Sloanea zuliaensis [1]: 23
Cucurbitacin E
- Cucurbitacin E (aelaterin), from roots of Wilbrandia ebracteata. Strong antifeedant for the flea beetle, inhibits cell adhesion [1] (also in e.g. Cucurbita andreana[7]): 27
- 22,23-Dihydrocucurbitacin E from Hemsleya endecaphylla, 9 mg/72 g,[6]: 8 and from roots of Wilbrandia ebracteata [1]: 28
- 22,23-Dihydrocucurbitacin E 2-glucoside from roots of Wilbrandia ebracteata [1]: 29
- Isocucurbitacin E, from Cucumis prophetarum [1]: 30
- 23,24-Dihydroisocucurbitacin E, from Cucumis prophetarum [1]: 31
Cucurbitacin F
- Cucurbitacin F from Elaeocarpus dolichostylus [1]: 33
- Cucurbitacin F 25-acetate from Helmseya graciliflora [1]: 34
- 23,24-Dihydrocucurbitacin F from Helmseya amabilis [1]: 35
- 25-Acetoxy-23,24-dihydrocucurbitacin F from Helmseya amabilis (hemslecin A) [1]: 36
- 23,24-Dihydrocucurbitacin F glucoside from Helmseya amabilis [1]: 40
- Cucurbitacin II glucoside from Helmseya amabilis [1]: 41
- Hexanorcucurbitacin F from Elaeocarpus dolichostylus [1]: 43
- Perseapicroside A from Persea mexicana [1]: 44
- Scandenoside R9 from Hemsleya panacis-scandens [1]: 45
- 15-Oxo-cucurbitacin F from Cowania mexicana [1]: 46
- 15-oxo-23,24-dihydrocucurbitacin F from Cowania mexicana [1]: 47
- Datiscosides B, D, and H, from Datisca glomerata [1]: 48–50
Cucurbitacin G
- Cucurbitacin G from roots of Wilbrandia ebracteata [1]: 52
- 3-Epi-isocucurbitacin G, from roots of Wilbrandia ebracteata [1]: 54
Cucurbitacin H
- Cucurbitacin H, stereoisomer of cucurbitacin G, from roots of Wilbrandia ebracteata [1]: 53
Cucurbitacin I

- Cucurbitacin I (elatericin B) from Hemsleya endecaphylla, 10 mg/72 g,[6]: 7 also from Ecballium elaterium,[1] Citrullus colocynthis,[1] Cucurbita andreana,[7] deters feeding by flea beetle [1]: 55
- Hexanorcucurbitacin I from Ecballium elaterium [1]: 56
- 23,24-Dihydrocucurbitacin I see Cucurbitacin L
- Khekadaengosides D and K from the fruits of Trichosanthes tricuspidata [1]: 57, 58
- 11-Deoxocucurbitacin I, from Desfontainia spinosa [1]: 59
- Spinosides A and B, from Desfontainia spinosa [1]: 61, 62
- 23,24-dihydro-11-Deoxocucurbitacin I from Desfontainia spinosa [1]: 60
Cucurbitacin J
- Cucurbitacin J from Iberis amara [1]: 69
- Cucurbitacin J 2-O-β-glucopyranoside from Trichosanthes tricuspidata [1]: 71
Cucurbitacin K
- Cucurbitacin K, stereoisomer of cucurbitacin J,[9]: 2 from Iberis amara [1]: 70
- Cucurbitacin K 2-O-β-glucopyranoside from Trichosanthes tricuspidata [1]: 72
Cucurbitacin L
- Cucurbitacin L, or 23,24-dihydrocucurbitacin I,[1]: 63 [9]: 1
- Brydioside A from Bryonia dioica [1]: 64
- Bryoamaride from Bryonia dioica [1]: 65
- 25-O-Acetylbryoamaride from Trichosanthes tricuspidata [1]: 66
- Khekadaengosides A and B from Trichosanthes tricuspidata [1]: 67–68
Cucurbitacin O
- Cucurbitacin O from Brandegea bigelovii [1]: 73
- Cucurbitacin Q 2-O-glucoside, from Picrorhiza kurrooa [1]: 76
- 16-Deoxy-D-16-hexanorcucurbitacin O from Ecballium elaterium [1]: 77
- Deacetylpicracin from Picrorhiza scrophulariaeflora [1]: 78
- Deacetylpicracin 2-O-glucoside from Picrorhiza scrophulariaeflora [1]: 80
- 22-Deoxocucurbitacin O from Wilbrandia ebracteata [1]: 83
Cucurbitacin P
- Cucurbitacin P from Brandegea bigelovii [1]: 74
- Picracin from Picrorhiza scrophulariaeflora [1]: 79
- Picracin 2-O-glucoside from Picrorhiza scrophulariaeflora [1]: 79
Cucurbitacin Q

- Cucurbitacin Q from Brandegea bigelovii [1]: 75
- 23,24-Dihydrodeacetylpicracin 2-O-glucoside from Picrorhiza kurrooa [1]: 82
- Cucurbitacin Q1 from Cucumis species, actually Cucurbitacin F 25-acetate [1]
Cucurbitacin R
- Cucurbitacin R is actually 23,24-dihydrocucurbitacin D.[1]
Cucurbitacin S
- Cucurbitacin S from Bryonia dioica [1]: 84'85
Cucurbitacin T
- Cucurbitacin T, from the fruits of Citrullus colocynthis [1]: 86
28/29 Norcucurbitacins
There are several substances that can be seen as derving from cucurbita-5-ene skeleton by loss of one of the methyl groups (28 or 29) attached to carbon 4; often with the adjacent ring (ring A) becoming aromatic.[1]: 87–130
Other
Several other cucurbitacins have been found in plants.[1]: 152–156, 164–165
Occurrence and bitter taste
Constituents of the colocynth fruit and leaves (Citrullus colocynthis) include cucurbitacins.[10][11] The 2-O-β-D-glucopyranosides of cucurbitacins K and L can be extracted with ethanol from fruits of Cucurbita pepo cv dayangua.[9] Pentanorcucurbitacins A and B can be extracted with methanol from the stems of Momordica charantia.[5] Cucurbitacins B and I, and derivatives of cucurbitacins B, D and E, can be extracted with methanol from dried tubers of Hemsleya endecaphylla.[6]
Cucurbitacins impart a bitter taste in plant foods such as cucumber, zucchini, melon and pumpkin.[12][13]
Research and toxicity
Cucurbitacins are under basic research for their biological properties, including toxicity and potential pharmacological uses in development of drugs for inflammation, cancer, cardiovascular diseases, and diabetes, among others.[1][2][3][12]
Pathologists found cucurbitacin in the stomach of a 79-year-old man who died in Bavaria, Germany, shortly after eating a casserole containing zucchini he had received from a neighbor.[14][15]
See also

- Goyaglycoside
- Hemslecin
- Mogroside
- Momordicine
- Momordicoside
- Neomogroside
- Scandenosides R1–R8, R10-R11[1]: 172–181
- Siamenoside I[1]: 182
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw Jian Chao Chen, Ming Hua Chiu, Rui Lin Nie, Geoffrey A. Cordell and Samuel X. Qiu (2005), "Cucurbitacins and cucurbitane glycosides: structures and biological activities" Natural Product Reports, volume 22, pages 386-399 doi:10.1039/B418841C
- ^ a b Alghasham, AA (2013). "Cucurbitacins - a promising target for cancer therapy". International Journal of Health Sciences. 7 (1): 77–89. doi:10.12816/0006025. PMC 3612419. PMID 23559908.
- ^ a b Kapoor, S (2013). "Cucurbitacin B and Its Rapidly Emerging Role in the Management of Systemic Malignancies Besides Lung Carcinomas". Cancer Biotherapy and Radiopharmaceuticals. 28 (4): 359. doi:10.1089/cbr.2012.1373. PMID 23350897.
- ^ a b Zhang, Y.; et al. (2014). "Biosynthesis, regulation, and domestication of bitterness in cucumber". Science. 346 (6213): 1084–1088. Bibcode:2014Sci...346.1084S. doi:10.1126/science.1259215. PMID 25430763.
- ^ a b c Chen, Chiy-Rong; Liao, Yun-Wen; Wang, Lai; Kuo, Yueh-Hsiung; Liu, Hung-Jen; Shih, Wen-Ling; Cheng, Hsueh-Ling; Chi-I, Chang (2010). "Cucurbitane Triterpenoids from Momordica charantia and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells". Chemical & Pharmaceutical Bulletin. 58 (12): 1639–1642. doi:10.1248/cpb.58.1639.
- ^ a b c d e f g Chen, Jian-Chao; Zhang, Gao-Hong; Zhang, Zhong-Quan; Qiu, Ming-Hua; Zheng, Yong-Tang; Yang, Liu-Meng; Yu, Kai-Bei (2008). "Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers of Hemsleya endecaphylla with HIV-1 Inhibitory Activity". J. Nat. Prod. 71 (1): 153–155. doi:10.1021/np0704396. PMID 18088099.
- ^ a b c d Halaweish, FT; Tallamy, DW (1993). "A new cucurbitacin profile for Cucurbita andreana: A candidate for cucurbitacin tissue culture". Journal of Chemical Ecology. 19 (6): 1135–1141. doi:10.1007/BF00987375. PMID 24249132.
- ^ Kupchan, S.Morris; Meshulam, Haim; Sneden, Albert T. (1978). "New cucurbitacins from Phormium tenax and Marah oreganus". Phytochemistry. 17 (4): 767–769. doi:10.1016/S0031-9422(00)94223-7.
- ^ a b c Wang, Da-Cheng; Pan, Hong-Yu; Deng, Xu-Ming; Xiang, Hua; Gao, Hui-Yuan; Cai, Hui; Wu, Li-Jun (2007). "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua"". Journal of Asian Natural Products Research. 9 (6): 525–529. doi:10.1080/10286020600782538.
- ^ Song, F; Dai, B; Zhang, H. Y.; Xie, J. W.; Gu, C. Z.; Zhang, J (2015). "Two new cucurbitane-type triterpenoid saponins isolated from ethyl acetate extract of Citrullus colocynthis fruit". Journal of Asian Natural Products Research. 17 (8): 813–8. doi:10.1080/10286020.2015.1015999. PMID 25761128.
- ^ Chawech, R; Jarraya, R; Girardi, C; Vansteelandt, M; Marti, G; Nasri, I; Racaud-Sultan, C; Fabre, N (2015). "Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad". Molecules. 20 (10): 18001–15. doi:10.3390/molecules201018001. PMID 26437392.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Kaushik, U; Aeri, V; Mir, S. R (2015). "Cucurbitacins – an insight into medicinal leads from nature". Pharmacognosy Reviews. 9 (17): 12–18. doi:10.4103/0973-7847.156314. PMC 4441156.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shang, Y; Ma, Y; Zhou, Y; Zhang, H; Duan, L; Chen, H; Zeng, J; Zhou, Q; Wang, S; Gu, W; Liu, M; Ren, J; Gu, X; Zhang, S; Wang, Y; Yasukawa, K; Bouwmeester, H. J.; Qi, X; Zhang, Z; Lucas, W. J.; Huang, S (2014). "Plant science. Biosynthesis, regulation, and domestication of bitterness in cucumber". Science. 346 (6213): 1084–8. Bibcode:2014Sci...346.1084S. doi:10.1126/science.1259215. PMID 25430763.
- ^ "Mann stirbt an Garten Zucchini". Retrieved 24 August 2015.
- ^ "Auf den Geschmack kommt es an". Retrieved 24 August 2015.
