X-gal: различия между версиями
| [отпатрулированная версия] | [отпатрулированная версия] |
Sirozha (обсуждение | вклад) Нет описания правки |
м r2.5.2) (робот добавил: en:X-gal |
||
| Строка 115: | Строка 115: | ||
[[de:5-Brom-4-chlor-3-indoxyl-β-D-galactopyranosid]] |
[[de:5-Brom-4-chlor-3-indoxyl-β-D-galactopyranosid]] |
||
[[en:X-gal]] |
|||
[[es:X-gal]] |
[[es:X-gal]] |
||
[[fr:X-gal]] |
[[fr:X-gal]] |
||
Версия от 14:54, 11 декабря 2010
| X-gal | |
|---|---|
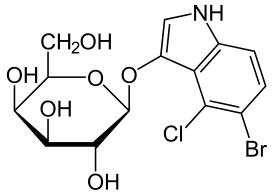 | |
| Общие | |
| Систематическое наименование |
5-бромо-4-хлоро-3-индоил-бета-D-галактопиранозид |
| Хим. формула | C14H15BrClNO6 |
| Физические свойства | |
| Молярная масса | 408,629 г/моль |
| Классификация | |
| Рег. номер CAS | 7240-90-6 |
| PubChem | 65181 |
| Рег. номер EINECS | 230-640-8 |
| SMILES | |
| InChI | |
| ChEBI | 75055 |
| ChemSpider | 58680 |
| Приведены данные для стандартных условий (25 °C, 100 кПа), если не указано иное. | |
X-gal (также BCIG от англ. bromo-chloro-indolyl-galactopyranoside) — органическое соединение, состоящее из галактозы, соединенной с индолом. Очень широко используется в качестве субстрата для бета-галактозидаз в генетической инженерии и молекулярной биологии.
Uses
Раздел в данный момент активно редактирует участник Sirozha.ru. |
[[Категория:Википедия:Ошибка выражения: неожидаемый оператор <, редактируемые прямо сейчас]]
Cloning
In gene cloning, X-gal is used to indicate whether a cell expresses the β-galactosidase enzyme, which is encoded by the lacZ gene, in a technique called blue/white screening.
X-gal is cleaved by β-galactosidase yielding galactose and 5-bromo-4-chloro-3-hydroxyindole. The latter is then oxidized into 5,5'-dibromo-4,4'-dichloro-indigo, an insoluble blue product. Thus, if X-gal and an inducer of β-galactosidase (usually IPTG) is contained within an agar medium on a culture plate, colonies which have a functional lacZ gene can easily be distinguished.
When a technique of cloning plasmid vector genes within bacterial cells is optimal, X-gal is used to visually locate yeast or E. coli colonies that have been transformed by the desired plasmid vector in a blue-white screen. E. coli bacteria, which cannot produce the enzyme β-galactosidase (coded by lacZ gene of the lac operon), are transformed by absorbing the plasmid vectors, which contain an insert in the lacZ open reading frame. After the transformation process, the bacteria is spread on nutrient agar plates, which mostly contain antibiotics as well. Most commercially available vectors contain an antibiotic-resistant gene. Successfully transformed bacteria has a truncated β-galactosidase gene, causing white colonies on the plate. Bacteria transformed by empty vectors, which does not contain an insert in the lacZ open reading frame, are now able to produce the enzyme β-galactosidase which can then cleave the X-gal present within the nutrient agar, resulting in a blue colony. Bacteria colonies that grew from bacteria that were not transformed does not contain the antibiotic-resistance, and thus, die. The plasmid vectors can also be coded to disrupt a different bacteria’s ability to produce β-galactosidase causing the desired bacteria colonies to grow to be white and non-transformed colonies to grow to be blue. This is the case with many commercially available cloning vectors, such as Promega’s pGem-T Vectors, which carry lacZα, a truncated form of β-galactosidase, and require specific E. coli hosts strains (such as DH5α) to achieve α-complementation.
Reporter
The lacZ gene may be used as a reporter in combination with growth media containing X-gal. In two-hybrid analysis for example, it is necessary to distinguish between those yeast or bacteria in which there is a successful interaction, leading to the binding of an activation domain to a promoter, and those in which there is not. If the promoter is linked to a lacZ gene, the production of β-galactosidase will be indicated by the production of blue pigment by colonies that host a successful interaction.[1] Due to its manual nature, this technique is limited to situations in which the number of colonies that must be distinguished is less than around 106.[1] The successful cleavage of X-gal also creates a noticeably foul odor due to the volatilization of indole.
Water testing
In addition to use in molecular biology, X-Gal is used to determine E. coli and coliform content in drinking water samples.
Примечания
- ↑ 1 2 Joung J, Ramm E, Pabo C (2000). "A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions". Proc Natl Acad Sci USA. 97 (13): 7382—7. doi:10.1073/pnas.110149297. PMC 16554. PMID 10852947.
{{cite journal}}: Википедия:Обслуживание CS1 (множественные имена: authors list) (ссылка)
