硒酸亚铁:修订间差异
外观
删除的内容 添加的内容
无编辑摘要 |
小 →top |
||
| (未显示2个用户的3个中间版本) | |||
| 第5行: | 第5行: | ||
| Name = 硒酸亚铁 |
| Name = 硒酸亚铁 |
||
| NameEn = Iron(II) selenate<BR>Ferrous selenate |
| NameEn = Iron(II) selenate<BR>Ferrous selenate |
||
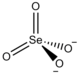
|CustomizedImage=[[File:Fe2+.svg]][[File:Selenate.png|79px]] |
|||
| ImageFile = |
|||
| ImageSize = |
|||
| ImageName = |
|||
| IUPACName = |
| IUPACName = |
||
| OtherNames = |
| OtherNames = |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo_Ref = |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo1_Ref = {{cascite|correct|CAS}} |
|||
| CASNo = 15857-43-9 |
| CASNo = 15857-43-9 |
||
| CASNo_Comment = 无水 |
| CASNo_Comment = 无水 |
||
| 第56行: | 第55行: | ||
}} |
}} |
||
'''硒酸亚铁'''是一种[[无机化合物]],为二价铁的[[硒酸盐]],化学式为FeSeO<sub>4</sub>。其五水合物的结构为[Fe(H<sub>2</sub>O)<sub>4</sub>]SeO<sub>4</sub>·H<sub>2</sub>O<ref>K. Heinzinger, G. Pálinkás, Hubertus Kleeberg. Interactions of Water in Ionic and Nonionic Hydrates: Proceedings of a Symposium in honour of the 65th birthday of W.A.P. Luck Marburg/FRG, 2.–3.4. 1987. Springer-Verlag Berlin Heidelberg, 1987</ref>。 |
'''硒酸亚铁'''是一种[[无机化合物]],为二价铁的[[硒酸盐]],化学式为FeSeO<sub>4</sub>。其五水合物的结构为[Fe(H<sub>2</sub>O)<sub>4</sub>]SeO<sub>4</sub>·H<sub>2</sub>O<ref>K. Heinzinger, G. Pálinkás, Hubertus Kleeberg. Interactions of Water in Ionic and Nonionic Hydrates: Proceedings of a Symposium in honour of the 65th birthday of W.A.P. Luck Marburg/FRG, 2.–3.4. 1987. Springer-Verlag Berlin Heidelberg, 1987</ref>。 |
||
==制备== |
==制备== |
||
硒酸亚铁可以通过饱和的[[硒酸钠]]和[[硫酸亚铁]]溶液在80℃混合,冷却至20℃后沉淀出来,用丙酮洗涤后于120℃干燥。<ref>Filonenko, E. N.; Vergeichik, E. N.. Preparation and analysis of cobalt (II) selenate and iron (II) selenate. ''Farmatsiya (Moscow)'', 1998. 47 (3): 36-37</ref> |
硒酸亚铁可以通过饱和的[[硒酸钠]]和[[硫酸亚铁]]溶液在80℃混合,冷却至20℃后沉淀出来,用丙酮洗涤后于120℃干燥。<ref>Filonenko, E. N.; Vergeichik, E. N.. Preparation and analysis of cobalt (II) selenate and iron (II) selenate. ''Farmatsiya (Moscow)'', 1998. 47 (3): 36-37</ref> |
||
: Na<sub>2</sub>SeO<sub>4</sub>(sat.) + FeSO<sub>4</sub> → Na<sub>2</sub>SO<sub>4</sub> + FeSeO<sub>4</sub> |
: Na<sub>2</sub>SeO<sub>4</sub>(sat.) + FeSO<sub>4</sub> → Na<sub>2</sub>SO<sub>4</sub> + FeSeO<sub>4</sub> |
||
铁和[[硒酸]]的反应也能得到硒酸亚铁,但是也有副反应:<ref name=Tutton>A E H Tutton. [http://rspa.royalsocietypublishing.org/content/royprsa/94/661/352.full.pdf Selenic Acid and Iron. - Reduction of Selenic Acid by Nascent Hydrogen and Hydrogen Sulphide. - Preparation of Ferrous Selenate and Double Selenates of Iron Group]. ''Proceedings of the Royal Society A'', 1918 , 94 (241) :352-361</ref> |
铁和[[硒酸]]的反应也能得到硒酸亚铁,但是也有副反应:<ref name=Tutton>A E H Tutton. [http://rspa.royalsocietypublishing.org/content/royprsa/94/661/352.full.pdf Selenic Acid and Iron. - Reduction of Selenic Acid by Nascent Hydrogen and Hydrogen Sulphide. - Preparation of Ferrous Selenate and Double Selenates of Iron Group] {{Wayback|url=http://rspa.royalsocietypublishing.org/content/royprsa/94/661/352.full.pdf |date=20180719222708 }}. ''Proceedings of the Royal Society A'', 1918 , 94 (241) :352-361</ref> |
||
: Fe + H<sub>2</sub>SeO<sub>4</sub> → FeSeO<sub>4</sub> + H<sub>2</sub>↑ |
: Fe + H<sub>2</sub>SeO<sub>4</sub> → FeSeO<sub>4</sub> + H<sub>2</sub>↑ |
||
: 3 Fe + 4 H<sub>2</sub>SeO<sub>4</sub> → 3 FeSeO<sub>4</sub> + Fe + 4 H<sub>2</sub>O |
: 3 Fe + 4 H<sub>2</sub>SeO<sub>4</sub> → 3 FeSeO<sub>4</sub> + Fe + 4 H<sub>2</sub>O |
||
| 第65行: | 第65行: | ||
硒酸亚铁还可以形成[[矾]],如(NH<sub>4</sub>)<sub>2</sub>Fe(SeO<sub>4</sub>)<sub>2</sub>·6H<sub>2</sub>O。<ref name=Tutton />三价铁的硒酸盐Fe<sub>2</sub>(SeO<sub>4</sub>)<sub>3</sub>也是已知的。<ref>G Giester, F Pertlik. Synthesis and crystal structure of iron(III) selenate(IV) trihydrate, Fe<sub>2</sub>(SeO<sub>3</sub>)<sub>3</sub>·3H<sub>2</sub>O. ''Journal of Alloys & Compounds'', 1994 , 210 (1-2) :125-128</ref> |
硒酸亚铁还可以形成[[矾]],如(NH<sub>4</sub>)<sub>2</sub>Fe(SeO<sub>4</sub>)<sub>2</sub>·6H<sub>2</sub>O。<ref name=Tutton />三价铁的硒酸盐Fe<sub>2</sub>(SeO<sub>4</sub>)<sub>3</sub>也是已知的。<ref>G Giester, F Pertlik. Synthesis and crystal structure of iron(III) selenate(IV) trihydrate, Fe<sub>2</sub>(SeO<sub>3</sub>)<sub>3</sub>·3H<sub>2</sub>O. ''Journal of Alloys & Compounds'', 1994 , 210 (1-2) :125-128</ref> |
||
亚铁的亚硒酸盐尚未有文献报道,对应的三价铁亚硒酸盐和焦亚硒酸盐已被制得。<ref>Pinaev, G. F.; Stukan, R. A.; Makarov, E. F.. Moessbauer effect in iron(3+) selenites. ''Zhurnal Neorganicheskoi Khimii'', 1977. 22 (7): 1731-1733</ref> |
亚铁的亚硒酸盐尚未有文献报道,对应的三价铁[[亚硒酸铁|亚硒酸盐]]和焦亚硒酸盐已被制得。<ref>Pinaev, G. F.; Stukan, R. A.; Makarov, E. F.. Moessbauer effect in iron(3+) selenites. ''Zhurnal Neorganicheskoi Khimii'', 1977. 22 (7): 1731-1733</ref> |
||
==参考文献== |
==参考文献== |
||
{{reflist}} |
{{reflist}} |
||
2022年1月13日 (四) 07:04的最新版本
| 硒酸亚铁 | ||
|---|---|---|
|
| ||
| 英文名 | Iron(II) selenate Ferrous selenate | |
| 识别 | ||
| CAS号 | 15857-43-9(无水) 70807-08-8(五水) | |
| 性质 | ||
| 化学式 | FeSeO4 | |
| 摩尔质量 | 198.808(无水) 288.888(五水) g·mol⁻¹ | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
硒酸亚铁是一种无机化合物,为二价铁的硒酸盐,化学式为FeSeO4。其五水合物的结构为[Fe(H2O)4]SeO4·H2O[1]。
制备
[编辑]硒酸亚铁可以通过饱和的硒酸钠和硫酸亚铁溶液在80℃混合,冷却至20℃后沉淀出来,用丙酮洗涤后于120℃干燥。[2]
- Na2SeO4(sat.) + FeSO4 → Na2SO4 + FeSeO4
- Fe + H2SeO4 → FeSeO4 + H2↑
- 3 Fe + 4 H2SeO4 → 3 FeSeO4 + Fe + 4 H2O
相关物种
[编辑]硒酸亚铁还可以形成矾,如(NH4)2Fe(SeO4)2·6H2O。[3]三价铁的硒酸盐Fe2(SeO4)3也是已知的。[4]
亚铁的亚硒酸盐尚未有文献报道,对应的三价铁亚硒酸盐和焦亚硒酸盐已被制得。[5]
参考文献
[编辑]- ^ K. Heinzinger, G. Pálinkás, Hubertus Kleeberg. Interactions of Water in Ionic and Nonionic Hydrates: Proceedings of a Symposium in honour of the 65th birthday of W.A.P. Luck Marburg/FRG, 2.–3.4. 1987. Springer-Verlag Berlin Heidelberg, 1987
- ^ Filonenko, E. N.; Vergeichik, E. N.. Preparation and analysis of cobalt (II) selenate and iron (II) selenate. Farmatsiya (Moscow), 1998. 47 (3): 36-37
- ^ 3.0 3.1 A E H Tutton. Selenic Acid and Iron. - Reduction of Selenic Acid by Nascent Hydrogen and Hydrogen Sulphide. - Preparation of Ferrous Selenate and Double Selenates of Iron Group (页面存档备份,存于互联网档案馆). Proceedings of the Royal Society A, 1918 , 94 (241) :352-361
- ^ G Giester, F Pertlik. Synthesis and crystal structure of iron(III) selenate(IV) trihydrate, Fe2(SeO3)3·3H2O. Journal of Alloys & Compounds, 1994 , 210 (1-2) :125-128
- ^ Pinaev, G. F.; Stukan, R. A.; Makarov, E. F.. Moessbauer effect in iron(3+) selenites. Zhurnal Neorganicheskoi Khimii, 1977. 22 (7): 1731-1733