Talk:Molecular vibration
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||
| ||||||||||||||||||
Comments
[edit]This is a nice pedagogical article. I only don't like "constant rotational motion", because — in contrast to constant translational motion — rotation gives inertial forces (Coriolis and centrifugal) that are not accounted for in this article. So, I would prefer "zero rotational motion" (quantum mechanically phrased: J = 0 states). Because the original author is obviously very knowledgeable, I don't want to fiddle around with his/her text. Please author, think about changing "constant rotational motion" to "zero rotational motion". --P.wormer 16:29, 28 March 2007 (UTC)
- Your comments are most welcome. I wanted to avoid using the term constant angular momentum, but I guess that is technically more correct Another way of expressing the main point would be to say that vibrational coordinates are defined with respect to a set of Cartesian axes that move and rotate with the whole molecule. I'll have to think about this later as I'm travelling abroad tomorrow. The Coriolis force comes into play in vibration-rotation spectra and Coriolis coupling constants can be used as additional data in normal coordinate analysis, but this is surely beyond the scope of an article like this? Centrifugal distortion is not really relevant here. Peter Gans, home page Petergans 18:52, 28 March 2007 (UTC)
- Constant angular momentum won't do either, unless the constant is zero. I agree that the centrifugal distortion is not important, but it is more a matter of principle. So, why don't you simply say that that the assumption is that there is no overall (external) motion? Or that the associated effects are neglected, which still is a very good approximation. (BTW I answered your earlier remark on my talk page).--P.wormer 20:18, 28 March 2007 (UTC)
Edits
[edit]I made a number of changes to the internal coordinates section of the article: [1] - I am a little concerned about this paragraph which I introduced:
- Ethene consists of 6 atoms and so has 3(6) - 6 = 12 normal modes of vibration. This set of normal modes can be used as the internal coordinates for the molecule. Note that the H-C-C angles cannot be used as internal coordinates as the angles at each carbon atom cannot all increase at the same time. Generally speaking, internal coordinates are chosen so that the coordinate system is not redundant.
It used to say:
- In ethene there are 12 internal coordinates: 4 C-H stretching, 1 C-C stretching, 2 H-C-H bending, 2 CH2 rocking, 2 CH2 wagging, 1 twisting. Note that the H-C-C angles cannot be used as internal coordinates as the angles at each carbon atom cannot all increase at the same time.
I'd like to make this clearer...thoughts? --HappyCamper 16:16, 29 March 2007 (UTC)
- Nevermind, completely incorrect thinking on my part here. Bottom line? Normal modes internal coordinates. How did I ever confuse them? Anyway, keep the text as it is. I'll get busy with getting an image of ethene marked with internal coordinates for this article. --HappyCamper 22:19, 1 April 2007 (UTC)
- HC, normal coordinates are just one set (out of infinitely many sets) of internal coordinates. They form a basis for a 3N-6 dimensional subspace (the internal space) of the total 3N dimensional atomic configuration space. But any other basis for this subspace forms a valid set of internal coordinates. Normal coordinates have the additional property that they are a solution of a generalized eigenvalue problem (the GF problem) and thus give uncoupled oscillators (an oscillator being a collective motion of a number of atoms). As I understand it (but Petergans may correct me if I am wrong) redundancy means that coordinates have components outside the internal space. Stated equivalently: redundant coordinates do not satisfy the center-of-mass conditions (nor the Eckart conditions). For example, if all 4 H-atoms in ethene bend to one and the same side of the molecule, while the carbons stay in place, the COM shifts to this side. COM conditions state that the COM must stay in place for any change in internal coordinates.--P.wormer 01:07, 2 April 2007 (UTC)
- Petergans, if you don't agree with the above, and consider this wrong, I would appreciate you putting it straight. I am arrogant enough to think that—if I see this incorrectly—there will be many readers who don't understand it either. Explaining this coordinate business clearly and correctly would be a great help to the Wikipedia readers!--P.wormer 01:07, 2 April 2007 (UTC)
- I think this sort of information should be added to the article. We've written so much about it already on talk pages, it would be a shame not to help other readers. It would be nice to add a list of historical applications. Peter's book has quite a nice collection of examples and illustrations. In any case, this is the image request here: [2] - it might take a little bit of time. We could annotate the image ourselves, but the image department is known for producing quality illustrations. --HappyCamper 01:40, 2 April 2007 (UTC)
- From my perspective the most important property of normal coordinates is that they are mutually orthogonal and hence "independent" as stated in the article. The relative atomic displacements are in the eigenvectors of GF which can be transformed into displacements in whatever coordinate system one wants, including a Cartesian coordinate system.
- independent is in quotation marks simply because it is a quotation!Petergans 12:57, 5 April 2007 (UTC)
- Why do you write quotes around independent? A well-known theorem of linear algebra states that orthogonal vectors are linearly independent. So normal coordinates are certainly linearly independent. However, should orthogonality not be defined in a generalized sense? The inverse of G plays the role of the overlap matrix S in quantum chemistry and orthonormality reads (in quantum chemistry): CT S C = E (the unit matrix). The columns of C contain the solutions of the generalized eigenvalue problem. I would expect the equivalent orthogonality relation to hold for the solutions of the GF equations. (If not I have to revise this article.)--P.wormer 16:58, 4 April 2007 (UTC)
- I was going to include the Centre Of Mass issue in the sub-section on Cartesian Coordinates by stating that the origin of the molecular axes is placed at the COM, with atomic Cartesian displacements paralell to the molecular axes. Of course this does not cover curvilinear coordinates used for some anharmonic systems, but that's getting very esoteric. This article should concentrate on fundamentals.
- regarding redundant coordinates, the corresponding eigenvalues are zero so the eigenvectors are indeterminate. They can be identified in the context of the molecular point group. Otherwise they can become hard to spot, particulary in compounds containing rings where there are geometrical constraints on simultaneous changes on bond lengths and angles. Let's not even think about compounds with a cage structure! Once they have been identified if they are simply omitted from G and F their dimension is then 3N-(5 or 6).Petergans 15:59, 4 April 2007 (UTC)
Spanish equivalent
[edit]If anyone can verify it, is es:Vibración molecular Spanish Wikipedia's version of this article? We can add interlanguage links if this is the case. --HappyCamper 01:50, 2 April 2007 (UTC)
- I read Spanish. This article is very supeficial, but could be quoted for the sake of completeness.Petergans 09:21, 3 April 2007 (UTC)
Redundant coordinates again.
[edit]I like to make a few discussion points about redundant coordinates. After agreement is reached the endresult may be included in the main article.
- On the Hc talk page HappyCamper gives a long summary of what is written about redundant coordinates in Ref. 5 of the main article (Petergans' book). This summary relies heavily on point group symmetry. However, a general theory should cover vibrations of molecules without symmetry. This is what I tried to do in my remark above.
- HC quotes that: A redundant coordinate is, by definition one in which there is no net displacement of the atoms. My definition above (valid for the H-C-C angles in ethene) could be rephrased thus: A coordinate is redundant when its change causes a simultaneous and equal displacement of all atoms of the molecule. (A redundant coordinate may give an overall translation and/or overall rotation of the molecule). I can reconcile the two definitions when I interpret the external motions either passively (HC and Petergans), or actively (my definition). In the passive interpretation the frame is rotated/translated by a change in a redundant coordinate and the atoms stay fixed in this frame (no net displacement). In the active interpretation the molecule itself is translated/rotated by changing a redundant coordinate.
- In the case of BF3 redundancy comes from the relation (valid in the planar case): α12+α23+α31=2π. (α12+α23+α31 is an internal coordinate of A'1 symmetry).
- In mathematical terms: a set of coordinates is redundant if (i) they are dependent or (ii) they are not fully within the internal space (this is the case if they do not satisfy the COM or Eckart conditions).
- The COM and Eckart conditions are satisfied by definition when I talk about molecular vibrations. Moreover there is a strong link with symmetry. Taking the BF3 example, changes in the three angles α12, α23 and α31 are used as a basis for bending vibrations as the projection operator is applied to a symmetrically equivalent set and the three angles are permuted by the 3-fold rotation operation. It is obvious that the angles between lines meeting at a point are related by a linear expression; the use of rocking and wagging coordinates is designed to be a simple way of avoiding the introduction of redundant coordinates.Petergans 12:57, 5 April 2007 (UTC)
- I would like to hear the opinion of HappyCamper and Petergans, or any other knowledgeable person, on this difference in definitions. In particular I would like to hear it if I am mistaken.
- This discussion belongs on this talk page, not on the talk page of HC.
--P.wormer 01:11, 3 April 2007 (UTC)
Rotation again
[edit]Thanks for these comments. I won't be able to do anything until I return to Leeds as I don't have my reference books here in Italy. In the meantime, how about this for an opening sentence?
A molecular vibration occurs when atoms in a molecule are in periodic motion relative to a set of axes that move and rotate with the molecule as a whole.
This will allow me to introduce later (adding a sub-section on Cartesian coordinates)the Coriolis effect in terms of a rotating frame of reference, as discussed in the Coriolis effect article.
I would prefer not to get into redundant coordinates. The problem with writing this sort of article is how to give a synoptic view without making unacceptable simplifications or getting into technical fine details. Ethene was chosen because it illustrates well all internal coordinates except out-of-plane; unfortunately it raises the issue of redundant coordinates. On the other hand redundant coordinates can be effectively ignored as the corresponding eigenvalues of GF are zero.Petergans 07:08, 3 April 2007 (UTC)
- I would replace "move" with "translate uniformly". If uniform translation is not the case, then an external force accelerates the translational motion of the molecule; we don't want that. The same goes for rotation: we don't want an external torque. Hence "rotate uniformly" (constant angular velocity). If you would like to omit Coriolis and centrifugal forces from the article you can say that the molecule (or, if you prefer, its body-fixed axes) do not rotate. In quantum calculations this is a very common assumption (total angular momentum J = 0).--P.wormer 14:33, 3 April 2007 (UTC)
- It is a pity that you want to avoid redundant coordinates, but you may be right, it is perhaps too technical. And the point that zero eigenvalues will appear is a good one. Although one must modify the usual generalized eigenvalue programs somewhat to account for possible linear dependencies.--P.wormer 14:33, 3 April 2007 (UTC)
- Maybe it's a good idea to write redundant coordinates. --HappyCamper 04:10, 4 April 2007 (UTC)
Images
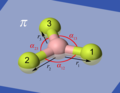
[edit]Hi everyone, check out these images:
Are these good for the article? --HappyCamper 22:53, 5 April 2007 (UTC)
- I leave it to Petergans to decide, because I am not very graphically minded and it is his article. What is π ? Out of plane angle?--P.wormer 00:47, 6 April 2007 (UTC)
- The idea is good. I would prefer to present the Ben with a set of consistent hand-drawn drafts. Putting in diagrams like these raises the issue of vibration coordinates being changes, for example in bond length, rather than properties of the molecule. I'll think about it and come up with some concrete proposals when I get back to Leeds.Petergans 06:51, 6 April 2007 (UTC)
- I'll ask Ben to see if he can make another image for us. --HappyCamper 18:38, 14 April 2007 (UTC)
Coordinate with infrared spectroscopy
[edit]This is wrong in principle. "Coordinate with vibrational spectroscopy" would be acceptible. Raman spectroscopy in all its forms cannot be ignored in the context of this article.Petergans 10:39, 12 April 2007 (UTC)
Mass-weighted coordinates
[edit]A reference to a free program has been added to the article. That program uses "mass-weighted coordinates". I have no knowledge of this method. My guess is that they refer to atomic Cartesian coordinates weighted in some way to the mass of the atom, that is, treating each atom as an oscillator. Can anyone help? Also your opinion as to whether this is a useful reference for this article will be welcome. It looks a bit abstruse to me and may not meet Wikepedia criteria of validation as the program is offered on an "as is" basis.Petergans 15:58, 7 April 2007 (UTC)
- See this section for an explanation of mass-weighted coordinates. Method is straightforward, only point of discussion is the choice of mass: nuclear, atomic, or effective mass? As regards to the link: I don't have problems with this as long is it is not commercial. Validation is a general problem with Wikipedia. Who validates any article? This is a matter of chance. The same goes for free programs. --P.wormer 18:50, 7 April 2007 (UTC)
Thanks for this. It also clarifies something that has been a matter of concern for some time which I can describe crudely as the conflict between the physicists' and chemist' points of view. In this instance the article to which you refer uses the term "internal coordinates" to describe any set internal to the molecule whereas in chemistry texts (including Wilson, Decius and Cross)the term applies specifically to changes in bond length etc. I don't just now see how to reconcile the different uses of the same name.
- As I see it, changes in valence coordinates (bond stretch, valence angle bending, etc.) almost always satisfy the six (linear) Eckart conditions. If they do not, we call them redundant (example: 4 H-C-C angles in ethene. Bending all four of them into the same direction shifts the center of mass—violates the translational Eckart conditions). This obeying of Eckart c.'s is a consequence of the fact that most valence coordinates are independent of the position and the orientation of the molecule (see this article). The GF method is based on linearized valence coordinates (Wilson s-vectors) and these also satisfy the Eckart conditions (are by the very definition internal coordinates). If my view is correct (but I don't exclude the possibility of flaws in my view) then the chemistry and physics definitions coincide.--P.wormer 18:21, 10 April 2007 (UTC)
Eckart conditions don't come into it, nor does the COM.
1) The 6 HCH angles in methane are a symmetrically eqivalent set and as such are a basis for the 6 irreducble representations A1+E+T2. The A1 coordinate is redundant. Using the projection operator all 6 angles change by the same amount. This is physically possible only when the changes are zero.
2) HCC angles in ethylene. The four angles are a basis for Ag+B1g+B2u+B3u. The Ag coordinate has all 4 angles change by the same amount; this is the same as both HCH angle changing by minus twice that amount, so it is not a separate coordinate. The B3u coordinate is redundant. Applying the projection operator all 4 angle changes are zero. B1g and B2u are rocking coordinates.
In general a redundant coordinate has a zero eigenvalue and this implies no internal movement of the atoms. In the case one uses 3N Cartesian coordinates, the six translational and rotational coordinates have zero displacement relative to the internal axes. In cases such as B2u the normal coordinates are such as to automatically adjust the atomic positions so that the COM does not move.Petergans 07:50, 11 April 2007 (UTC)
- Petergans, you say that Eckart conditions don't come into it. However, you do speak of internal axes, is it not the case that these axes constitute an Eckart frame? (Recall that a displacement vector expressed with respect to an Eckart frame automatically satisfies the 6 Eckart conditions, the first 3 Eckart conditions being the COM conditions). Another question: is a set of linearized internal coordinates (such as they enter the GF method) containing a redundant coordinate linearly dependent?--P.wormer 22:46, 11 April 2007 (UTC)
By definition redundant valence coordinates cannot involve a change of COM. As I showed above, a redundant valence coordinate has valence displacements equal to zero, which in turn means that the vibration frequency is zero and the eigenvalue of GF is zero.
If there are two redundant valence coordinates they are both zero vectors, so the issue of linear dependency between them does not arise.Petergans 10:32, 12 April 2007 (UTC)
New draft
[edit]On my user page. Comments will be welcomePetergans 18:12, 14 April 2007 (UTC)
- I put it in /draft. --HappyCamper 18:28, 15 April 2007 (UTC)
- Yes, I think the Wikipedian is working on doing the image. So, I think it's on the way. I have to run for now, but just glancing through the article, can we add a little note about how to deal with caged compounds? --HappyCamper 14:30, 22 April 2007 (UTC)
Newtonian Model Inconsistency
[edit]In the section regarding a Newtonian SHM model of vibration the mass for homonuclear diatomic molecules is given as the total molecular mass, while for the heteronuclear case the reduced mass should be used. However, when applying the reduced mass formula to the homonuclear case the mass found is 1/4 that of the total molecular mass, as it should. So I believe there is an error here. Someone more well versed in the topic, or who has access to the source material should confirm. Thanks. <navaburo> --155.246.141.129 (talk) 15:54, 27 June 2008 (UTC)
It appears that in Petergans's draft only the reduced mass formula is given, which takes care of the above issue in my opinion. --155.246.141.129 (talk) 16:00, 27 June 2008 (UTC)
Possible duplicate article
[edit]Atom vibration redirects to this article, while Atom vibrations is a separate one. Please check whether these articles should be merged - and if not, whether it would be more sensible to redirect "Atom vibration" to "Atom vibrations". -- 91.48.252.88 (talk) 15:33, 24 November 2011 (UTC)
- I agree with you on merging Atom vibrations into this article and I've made a merge proposal. I believe that the issue with the duplicate pages has already been fixed. TripleShortOfACycle (talk - contribs) - (she/her/hers) 13:44, 14 January 2021 (UTC)
Redundant diagram for CH2 group
[edit]A new animated diagram was added yesterday by PakpongICCH444 showing the asymmetrical stretching of methylene group of ethylene. It is a very nice diagram but there are two problems.
- It is redundant because it essentially duplicates the second of six diagrams in the next section for the six vibrations of a methylene group.
- It is labelled as a diagram for ethylene. However since ethylene consists of two identical methylene groups bonded to each other, there are actually two asymmetrical stretching modes corresponding to the in-phase and out-of-phase combinations of the asymmetrical stretches of the two groups. It is true that the six modes show movement of only one CH2 group; this is appropriate for a CH2 group attached to a much larger molecule which is approximately stationary.
In view of these problems, is there a reason for keeping the diagram in the article? Dirac66 (talk) 21:29, 5 November 2015 (UTC)
- I removed it, but if you change your mind, revert me. A number of students are in the process of adding content to Wikipedia pages but almost everything so far has been fairly redundant or obvious figures. I guess they learn something from making these drawings, but I have not seen any improvements yet. Part of the problem is that the class is targeting fairly mature Wikipedia articles, where there are few opportunities for novices. Hope springs eternal.--Smokefoot (talk) 23:05, 5 November 2015 (UTC)
grams
[edit]In my opinion, formulas as vi=sqr(k/m)*const are best written without the units - a specification that generally causes wrong ideas in the students about the general validity of the equation. Perhaps my students are *** and I do question the approach used.
But if you specify the dimensions of ni and k, do not forget that of m. 151.29.59.56 (talk) 13:56, 26 July 2022 (UTC)
The intensities should be proportional to the square of the changes of dipole and polarizability.
The statement to the fourth-power of the wavelength refers only to the Raman, but it is easy to misunderstand that refers to both. I suggest to split on two lines. — Preceding unsigned comment added by 151.29.59.56 (talk) 19:26, 26 July 2022 (UTC)
The statement
The normal modes are determined by applying group theory, and projecting the ...
should hold only if there is at most one mode for each symmetry. — Preceding unsigned comment added by 151.29.59.56 (talk) 19:31, 26 July 2022 (UTC)
Hash
[edit]Hash is a term that is commonly used in the world of computer science and cryptography. It refers to a mathematical function that takes an input (or "message") and produces a fixed-size string of characters, which is typically a sequence of numbers and letters. This string is known as the hash value or simply the hash. Pgmcorp (talk) 10:29, 4 March 2024 (UTC)