Tetrolic acid

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
But-2-ynoic acid | |
| Other names
2-Butynoic acid
Butynoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.815 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3C≡CCO2H | |
| Molar mass | 84.074 g·mol−1 |
| Density | 0.9641 g/cm3[1] |
| Melting point | 78 °C (172 °F; 351 K)[1] |
| Boiling point | 203 °C (397 °F; 476 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrolic acid (2-butynoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3−C≡C−CO2H. Salts and esters of tetrolic acid are known as tetrolates.
History
[edit]The first reported synthesis[2] of tetrolic acid is believed to be by German chemist Johann Georg Anton Geuther in 1871 as part of his work investigating the derivatives of ethyl acetoacetate.
Production
[edit]Tetrolic acid is manufactured[3] on a commercial scale by treatment of propyne with a strong base (to form an acetylide), followed by carbon dioxide:
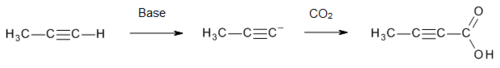
Strong bases such as n-BuLi[4] and NaNH2[5] can be used.
Properties
[edit]Tetrolic acid is highly soluble in polar solvents (water, ethanol) and can be recrystallized from non-polar solvents (such as heptane, hexane or toluene). The compound is a white crystalline solid which can exist in two polymorphous crystalline forms.[6]
The proton nuclear magnetic resonance (1H-NMR) spectrum in deuterated dimethyl sulfoxide shows a characteristic singlet peak at 1.99 ppm corresponding to the –CH3 protons.
Tetrolic acid sublimes at temperatures above 20°C, and should ideally be stored in a sealed container in a refrigerator.[7]
Accelerated rate calorimetry (ARC) showed exothermic onset from 135 °C, precluding short-path distillation as a means of purification.[7]
References
[edit]- ^ a b c Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 3.88. ISBN 1-4398-5511-0.
- ^ Geuther, A. (1871). "Ethyldiacetic Acid and some of its Derivatives". J. Chem. Soc. 24: 812–837. doi:10.1039/JS8712400808.
- ^ Smith, W. (1973) "Preparation of tetrolic acid" U.S. patent 3752848A
- ^ Hartzoulakis, Basil; Gani, David (1994). "Synthesis of (2S, 3R)- and (2S, 3S)-3-methylglutamic acid". J. Chem. Soc., Perkin Trans. 1. 1994 (18): 2525–2531. doi:10.1039/P19940002525.
- ^ Kauer, J. C.; Brown, M. (1962). "Tetrolic Acid (2-Butynoic Acid)". Organic Syntheses. 42: 97. doi:10.15227/orgsyn.042.0097; Collected Volumes, vol. 5, p. 1043.
- ^ Flakus, Henryk T.; Hachuła, Barbara (2008). "Effects of "excessive" exciton interactions in polarized IR spectra of the hydrogen bond in 2-butynoic acid crystals: Proton transfer induced by dynamical co-operative interactions involving hydrogen bonds". Chemical Physics. 345 (1): 49–64. Bibcode:2008CP....345...49F. doi:10.1016/j.chemphys.2008.01.035.
- ^ a b Golden, M. (2019). "Thermal Stability of 2-Butynoic Acid (Tetrolic acid)". Org. Process Res. Dev. 23, (5) (5): 1101–1104. doi:10.1021/acs.oprd.9b00106. S2CID 146028375.
